Improved health literacy means many couples want every test available to “ensure” their babies are healthy
The aim of prenatal screening and diagnosis is to identify chromosomal anomalies, genetic conditions and structural abnormalities in the fetus prior to birth.
The screening and diagnostic options involve a range of non-invasive and invasive tests at different stages of pregnancy, and have advanced in leaps and bounds over the last 40 years, in parallel with significant social and demographic changes over that time.
In Australia in 2013, the average age of women who gave birth was 30.1 years, compared with 29.5 in 2003, while the proportion of mothers aged 35 and over increased from 19% in 2003 to 22% in 2013, and the average age of first-time mothers also increased, from 27.8 years in 2003 to 28.6 in 2013.1
Forty years ago, maternal age was the only screening tool available to identify pregnancies at risk for chromosomal anomalies. Since then, the availability of increasingly cheap and accurate non-invasive prenatal tests has meant that women of all ages can now have improved prenatal testing options.
When faced with the many options for prenatal testing, some couples may feel overwhelmed, while clinicians may find it challenging to keep up with all the details of the new tests. But counselling is vital to ensure couples are fully aware of the testing process from physical, emotional, practical and financial perspectives.
The first and most important question to ask is: should the couple consider prenatal testing at all? Some couples never discuss the issue until they get pregnant, and many have never considered the possibility of having a baby with a chromosome problem, such as Down syndrome, or a birth defect, such as cleft lip.
On the other hand, these days improved health literacy means that many couples are informed and proactive, and some want every test available to ensure their babies are healthy.
The next question relates to which test/s to have, and whether these should be screening or diagnostic tests. By definition, a screening test surveys a population to identify those individuals at increased risk of having a certain condition, while a diagnostic test determines whether an individual has a particular condition. In the context of prenatal testing, screening tests include ultrasound scans, biochemical screening and non-invasive prenatal testing (NIPT), while diagnostic tests are chorionic villus sampling (CVS) and amniocentesis.
Finally, when talking to couples about testing, there are the practical issues of cost and access, which will be influenced to a large degree by the patient’s socioeconomic status. Many high-quality ultrasound services and other tests are not Medicare rebatable and therefore unaffordable for some patients.
Of course, just because a test is available doesn’t mean it should be performed, and couples embarking on prenatal testing need to do so with their eyes wide open to avoid ending up on a nightmarish rollercoaster ride when they are referred for “all the tests”.
To this end, pre-test counselling is vital so that couples understand the maternal age-related chances of a baby with a major chromosomal problem, and can then weigh these up against the risks and benefits of the various tests on offer.
Couples need to understand exactly what the tests can and cannot detect, and, most fundamentally, exactly why they are having the test. Difficult questions need to be answered at this stage: would they consider termination of a pregnancy if a fetus was diagnosed with Down syndrome? Would the diagnosis inform management and planning for delivery if a fetus with Down syndrome was also found to have a major cardiac defect requiring neonatal cardiac surgery?
This article considers two common case scenarios and the range of prenatal testing options available for both.
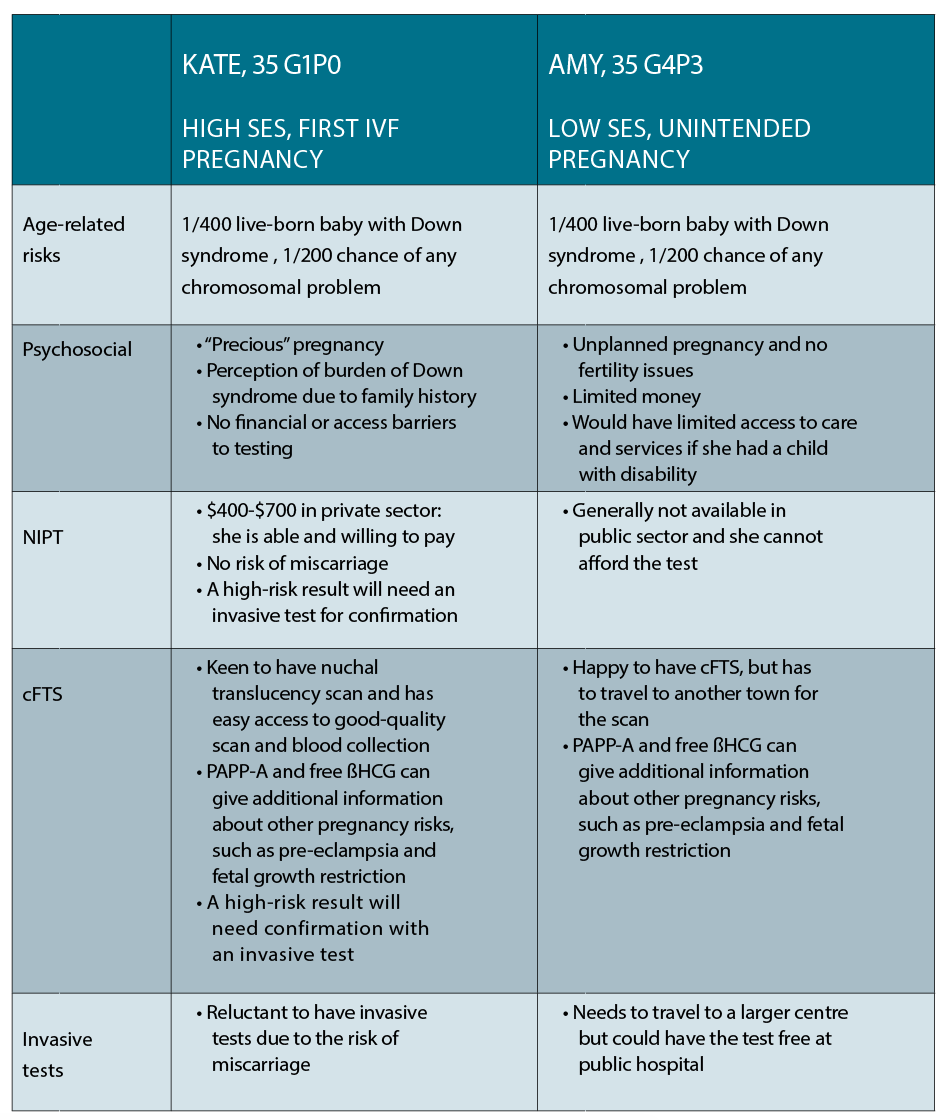
Case 1
Kate is a 35-year-old corporate lawyer living in the inner city, who has conceived her first pregnancy on her fourth cycle of IVF. She is now 10 weeks pregnant and wants to know the options available to her for prenatal diagnosis. She has a cousin with Down syndrome and is very concerned about her own risk of having a baby with the same condition.
Case 2
Amy is also 35 years old and living in a small town in NSW. She and her partner have three young children and are struggling financially, and she is 10 weeks into an unintended pregnancy. She wants to know her options for prenatal testing as one of her friends recently gave birth to a child with Down syndrome.

NON-INVASIVE TESTING
An early first trimester, or dating, ultrasound is performed between seven and 12 weeks, and is useful in confirming the presence of a viable pregnancy and determining if there is a multiple pregnancy. It also allows accurate dating of gestation, helps in excluding an ectopic pregnancy and localising the placenta. It is not performed to detect birth defects.
A nuchal translucency scan is performed between 11 weeks and three days, and 13 weeks and six days (fetal crown rump length of 45-84mm), ideally as part of “first trimester combined screening”. Nuchal translucency is a fluid-filled space at the back of the fetal neck which can be measured by ultrasound. It is well-recognised that the wider the nuchal translucency, the greater the risk of fetal anomalies, including chromosomal problems such as Down syndrome, as well as structural cardiac defects and some single gene disorders.
To date, the recommendation has been that the nuchal translucency scan should be done in conjunction with a maternal biochemical screening as a combined first trimester screen (cFTS), which measures PAPP-A and free ßHCG to improve Down syndrome detection rates.
Alone, the nuchal translucency scan has a detection rate of around 65% to 70% for Down syndrome, and with the addition of serum markers such as PAPP-A and free beta-HCG, both reported as multiples of the median (MoMs), this increases to around 90% with a false positive rate of 5%.
In most instances, these tests will be reassuring and offer couples peace of mind without putting the pregnancy at risk,
which can occur with invasive tests such as CVS or amniocentesis.
With the availability of non-invasive prenatal testing, debate has arisen as to the value of combined first trimester screening for Down syndrome, although many argue that the 12-week scan still has a role to play in identifying structural anomalies and other problems that might adversely affect the pregnancy outcome.2
Fetal morphology ultrasound is performed at 18 to 20 weeks, and can detect up to 50% of major structural anomalies. It is not recommended as a primary screening test for Down syndrome. The sensitivity of the scan in detecting malformations is influenced by a number of factors, including the nature of the malformation, the skill and experience of the operator, quality of the ultrasound machine, the maternal body habitus and position of the placenta.
NON-INVASIVE PRENATAL TESTING
A fetal genetic sample detectable in maternal blood, which can be tested for accurate prenatal diagnosis, has long been the holy grail of prenatal testing. Improvements in DNA technology have eventually led to the ability to isolate and measure circulating fetal DNA in maternal blood.3
Around 10% to 15% of DNA in maternal blood is fetal in origin, and comes from intact fetal cells as well as circulating cell-free fetal DNA (ccffDNA), derived predominantly from the breakdown of placental cells. The ccffDNA is cleared within hours of delivery from the maternal circulation, and fetal DNA detected during a pregnancy, therefore, represents DNA from the current fetus.
NIPT involves measuring the cell-free chromosome fragments, and using the quantitative differences to distinguish aneuploidy pregnancies from those that are not affected. For example, foetuses with Down syndrome will have a measurable and statistically significant increase in the number of chromosome 21 fragments.
However, like chorionic villus sampling, ccffDNA reflects placental rather than fetal DNA, which must be taken into account when counselling and discussing potential invasive test options. This may return cytogenetically ambiguous results caused by factors such as placental mosaicism.
Numerous biotechnology companies have launched different NIPT platforms with euphemistic names such as Harmony and Panorama, enabling large-scale non-invasive prenatal testing for fetal aneuploidy, such as trisomy 13, 18 and 21, and common sex chromosome anomalies, such as Turner syndrome.
The main advantages of NIPT are that it is non-invasive, available from nine weeks’ gestation, and has high sensitivity and specificity, although by definition it is a screening rather than a diagnostic test. However, around 25% of chromosomal anomalies will not be detected by NIPT, and therefore invasive testing should be offered to women at increased risk of a chromosomal anomaly, for example in the case of increased nuchal translucency measurement or cFTS, or those with a structural anomaly detected on ultrasound.4
There’s also a practical drawback in the Australian setting as NIPT is not publicly funded, leading to inequity of access. It is not yet established how NIPT should be incorporated into routine prenatal screening practice, or what the most cost-effective way is to do this.
Many experts currently advocate the contingent screening model whereby all women have cFTS, and those at high risk of Down syndrome (>1/50) are offered invasive testing; those at intermediate risk between 1/50 and 1/300 are offered a choice of either NIPT or invasive testing; and those at low risk (<1/300) are reassured and not offered further testing.5
INVASIVE OR DIAGNOSTIC TESTING
Over the last few years, the number of invasive prenatal testing procedures has declined significantly, mainly due to the availability of improved non-invasive testing.6 The main indications for performing invasive testing now are for prenatal diagnosis of single gene (Mendelian) disorders, rather than for detecting aneuploidy.
Chorionic villus sampling is an invasive test performed at 11 to 13 weeks of pregnancy, either via a trans-vaginal or trans-abdominal approach, depending on the preference of the operator and the location of the placenta. The procedure-related miscarriage rate is low (<1%) but nevertheless remains a barrier for some women, especially in those with a history of infertility or pregnancy loss.
There is a 1% chance of obtaining an inconclusive result with CVS. This is usually due to confined placental mosaicism, a well-recognised phenomenon that can be associated with low PAPP-A and adverse pregnancy outcomes, including fetal growth restriction and early pregnancy loss, even if the fetus has a normal karyotype. When the results of CVS are ambiguous, further testing – generally amniocentesis – is performed to clarify whether the chromosomal anomaly is truly present in the fetus, or is confined to the placenta.
Amniocentesis is performed after 15 weeks and is also associated with a small (<0.5%) risk of miscarriage. Amniocentesis is considered the gold standard, in that the fetal cells obtained from amniotic fluid are derived from several fetal tissues including the urinary tract and skin, and are thus more truly representative of the fetal karyotype than those obtained from the placenta. The main disadvantage of amniocentesis over CVS is the later timing, and hence more advanced gestation, by the time results are received.
Currently, most diagnostic labs have moved away from cytogenetic analysis (karyotype) as a standard test and have adopted molecular techniques, including quantitative fluorescent polymerase chain reaction (QF-PCR). QF-PCR is cheaper and less labour-intensive than standard karyotype analysis, and results are obtained more rapidly (within 24-48 hours compared with 10-14 days) as the technique does not require fetal cells to be cultured.
While QF-PCR is able to diagnose the common aneuploidies involving chromosomes 13, 18, 21 and the sex chromosomes, it cannot detect other rare chromosome anomalies. Also, it cannot determine whether a trisomy is due to non-disjunction (i.e. common, age-related aneuploidy) or translocation, which is rarer but of concern, as it may be inherited and associated with increased recurrence risk in future pregnancies.
Chromosome microarray, also known as molecular karyotyping, is well-established as one of the main investigations used to assess babies and children with structural anomalies and intellectual disability. It analyses chromosomes at a much higher resolution (generally <2.5kb or 250,000 base pairs) compared to the 5MB to 10MB resolution of a standard karyotype, or cytogenetic study, to detect small chromosomal duplications and deletions.
Increasingly, microarray is being used in the prenatal setting, where it can yield results that are difficult to interpret, referred to as “variants of uncertain significance”. For this reason, chromosome microarray should be offered only with appropriate pre- and post-test counselling, and only in situations where it is clinically indicated. In high-risk pregnancies where a structural anomaly or increased nuchal translucency measurement has been detected, microarray has been shown to improve diagnostic yield by around 6%.7
Preimplantation genetic diagnosis is now available for patients at increased risk of either aneuploidy or a single gene disorder. They are now able to undergo IVF and have embryos biopsied and tested via preimplantation genetic diagnosis on day five, prior to embryo transfer into the uterus.
PGD is increasingly being used by couples that wish to avoid a pregnancy affected with a particular genetic condition or chromosomal anomaly. While not 100% accurate, PGD can significantly reduce the risk of having an affected pregnancy, and thus the need to consider termination of pregnancy.
There are two main types of preimplantation genetic diagnosis:
Testing for aneuploidy: this is used for couples at risk of aneuploidy because of advanced maternal age, parental balanced translocation, recurrent miscarriage and implantation failure;
Testing for a single gene disorder: this is used in couples at risk of having a child with a single gene disorder, including autosomal recessive, X-linked or autosomal dominant conditions. In these situations, the specific molecular or DNA diagnosis needs to identified, before PGD can be offered.
It is vital that couples considering IVF and PGD are referred for appropriate counselling as they need to be made fully aware of the physical, emotional and financial point relevant to these procedures.
Dr Kennedy is Director MotherSafe RHW and Conjoint Lecturer School of Women’s and Children’s Health at UNSW
References:
1. AIHW Australia’s mothers and babies 2013 in brief
2. McLennan A, Palma-Dias R, da Silva Costa F, et al. Noninvasive prenatal testing in routine clinical practice- an audit of NIPT and combined first-trimester screening in an unselected Australian population. ANZJOG 2016; 56:22-28.
3. Lo YMD, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CWG, Wainscoat JS. 1997. Presence of fetal DNA in maternal plasma and serum. Lancet 350, 485–4874:
4. Petersen OB1, Vogel I, Ekelund C, Hyett J, Tabor A. Potential diagnostic consequences of applying non-invasive prenatal testing: population-based study from a country with existing first-trimester screening. Ultrasound Obstet Gynecol. 2014 Mar;43(3):265-71.
5. Hui L, Hyett J Noninvasive prenatal testing for trisomy 21; challenges for implementation in Australia. ANZJOG 2013; 53(5):416-424.
6. Hui L, Muggli EE, Halliday JL. Population-based trends in prenatal screening and diagnosis for aneuploidy: a retrospective analysis of 38 years of state-wide data. BJOG 2016; 123(1):90-7
7. Wapner RJ et al Chromosomal microarray versus karyotyping for prenatal diagnosis N Engl J Med 2012; 367:2175-2184