The stalled rollout of the cancer registers has certainly been a debacle, but there is a silver lining
From May 1, GPs will be able to offer either a Medicare-rebateable liquid-based cytology test or a Pap smear test as cervical cancer screening under the government’s new deal to manage the HPV screening delay.
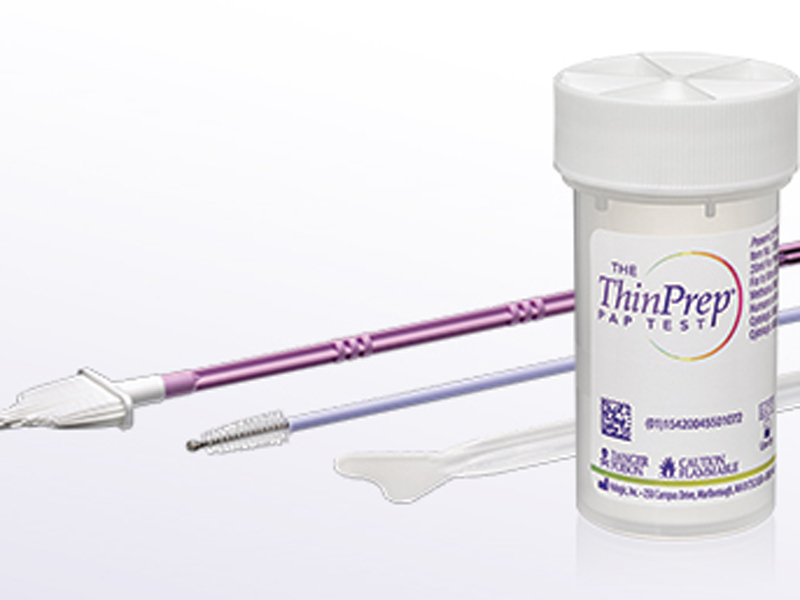
Instead of using ThinPrep or Sure Path as an additional, patient-funded test, GPs will be able to use the liquid-based cytology test as an alternative to the traditional Pap smear, but the Medicare rebate will only be available for one as part of the cervical cancer screening, a spokeswoman from the Department of Health told The Medical Republic.
The new Medicare rebate for the test was one of the stop-gap measures introduced in an attempt to deal with potentially ballooning wait times for pathology test results caused by the reduced number of cytologists being employed in anticipation of screening changes.
Last week, Department of Health officials told a Senate Estimates Community Affairs legislation committee that they would be expecting pathology labs to perform half of the tests using conventional Pap smear slides and half with liquid-based cytology tests, which include ThinPrep and SurePath.
The funding of the liquid-based cytology tests will benefit women who are already having them done and paying out-of-pocket for them.
In addition, it is hoped that the uptake of these less labour-intensive tests will reduce the backlog of Pap smears test results, which are currently reaching delays of up to three weeks.
Health officials said another $16.5 million would be outlaid by the Department of Human Services during the interim.
Pathology laboratories will get $3 million to compensate for the disruption, and to help retain staff who had been preparing for a May termination.
The other $13.5 million will go to the new Medicare rebates, upping the Pap smear rebate from $19.50 to $28, and introducing a new $36 rebate for liquid-based cytology tests.
At the time of publishing, it was expected that guidelines would soon be developed to provide further information on which tests should be ordered for which patients.
Associate Professor Charlotte Hespe, head of general practice at the University of Notre Dame, has been frustrated at the poor management and communication around the delay but said she welcomed the introduction of the new rebate.
“The advantage of changing to the fluid, as I see it, is that it will be a nice changeover period so that GPs can get used to putting the cells straight into a fluid-based medium and sending that off, instead of doing a slide,” she said.
Using this method, the same fluid could be used to do a HPV test if the results came back abnormal, she said.
“Although they are not changing over to the new system and register, we’re actually being given access to a Medicare item for a similar technology that is going to be being used.”
However, for the next two months GPs will need to do the Pap smears as normal.
Although they are not changing over to the new system and register, we’re actually being given access to a Medicare item for a similar technology that is going to be being used.
The health department and the RACGP have also urged women to continue getting a Pap smear at two-yearly intervals until the new register is officially introduced on 1 December.
The shock decision to delay the introduction of the new national bowel and cervical cancer screening registers has been met with dismay and frustration by doctors and stakeholders, who had little-to-no warning of the hold-up.
As details emerged that the government knew about the potential delays before Christmas, but only announced them last week, the government has been scrambling to assure doctors and clinicians that everything will run as normal.
Professor Hespe expressed annoyance at the lack of education and communication provided by the government, and pointed to a recent Change.org petition opposing the planned May 1 changes to cervical screening, which garnered more than 70,000 signatures, as evidence of how the project had been poorly explained.
“The petition really goes to the heart of it,” she said. “If people don’t know what a change means, you engender fear.”
The Shadow Minister for Health, Catherine King, has called the handling of the rollout a disgrace and said the government showed a lack of respect to women by keeping information about the delays a secret for two months.
“It’s disappointing that the complexity of the project was not scoped adequately prior to communicating the rollout date, in the first instance,” Dr Magdalena Simonis, co-chair of RACGP’s Women In General Practice, said.
Although there was a silver lining for the new December 1 rollout date, which was that the government had more time to properly prepare the community and to provide GPs with clear and uniform information for patients, she said.