The first reversal agent for the novel oral anticoagulant dabigatran has been approved by the TGA
The first reversal agent for the novel oral anticoagulant dabigatran has been approved by the TGA
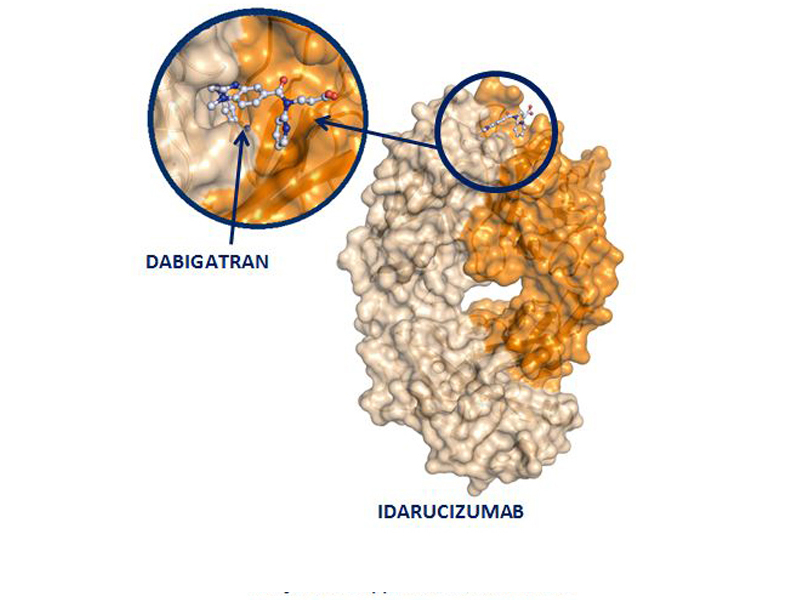
Idarucizumab, (Praxbind), is a monoclonal antibody that reverses the anticoagulant effect by binding specifically to dabigatran (Pradaxa). It will not reverse the effect of other newer anticoagulants, such as rivaroxaban and apixaban.
The drug is given intravenously as two consecutive infusions over five to ten minutes, or as a bolus injection, according to the TGA, and is now available in Australian hospitals.
The drug is not available on the PBS and will cost around $3,000 per course.
Idarucizumab is indicated for patients treated with dabigatran when rapid reversal of its anticoagulant effects is required for emergency surgery/urgent procedures or in life-threatening or uncontrolled bleeding, the TGA says.
Another reversal agent for NOACs was shown to be effective in a study in the NEJM last November, but is behind idarucizumab in the approval process.
Andexanet alfa is a recombinant protein designed to reverse the anticoagulant effect in patients treated with oral or injectable Factor Xa inhibitors. It is specifically designed to reverse the anticoagulant activity of both direct and indirect Factor Xa inhibitors.
So far, the lack of specific reversal agents has likely been a factor in the limited uptake of the novel oral anticoagulants (NOACs), given the potential for uncontrolled bleeding.