Haematologist Dr Ross Salvaris presents a hypothetical case study.
Follicular lymphoma is the most common indolent non-Hodgkin lymphoma (NHL), and survival has improved with the use of anthracycline-containing chemotherapy and the anti-CD20 monoclonal antibody rituximab.
Currently, the median survival is nearly 20 years1. However, most patients experience multiple relapses and require numerous treatments over their lifetime. Furthermore, 20% of patients with follicular lymphoma relapse within two years of their initial immunochemotherapy and have a poor prognosis1.
In patients with follicular lymphoma who have failed previous lines of treatment or are chemotherapy refractory, there is a need for non-chemotherapy agents with alternate mechanisms of action. This review will highlight three classes of novel agents with promising response rates in patients with relapsed/refractory follicular lymphoma.
Importantly, these therapies have unique adverse events and an understanding of their prevention and management is essential.
Case vignette
A 53-year-old man presented for umbilical hernia surgery and was found to have an asymptomatic 13.5 cm abdominal mass.
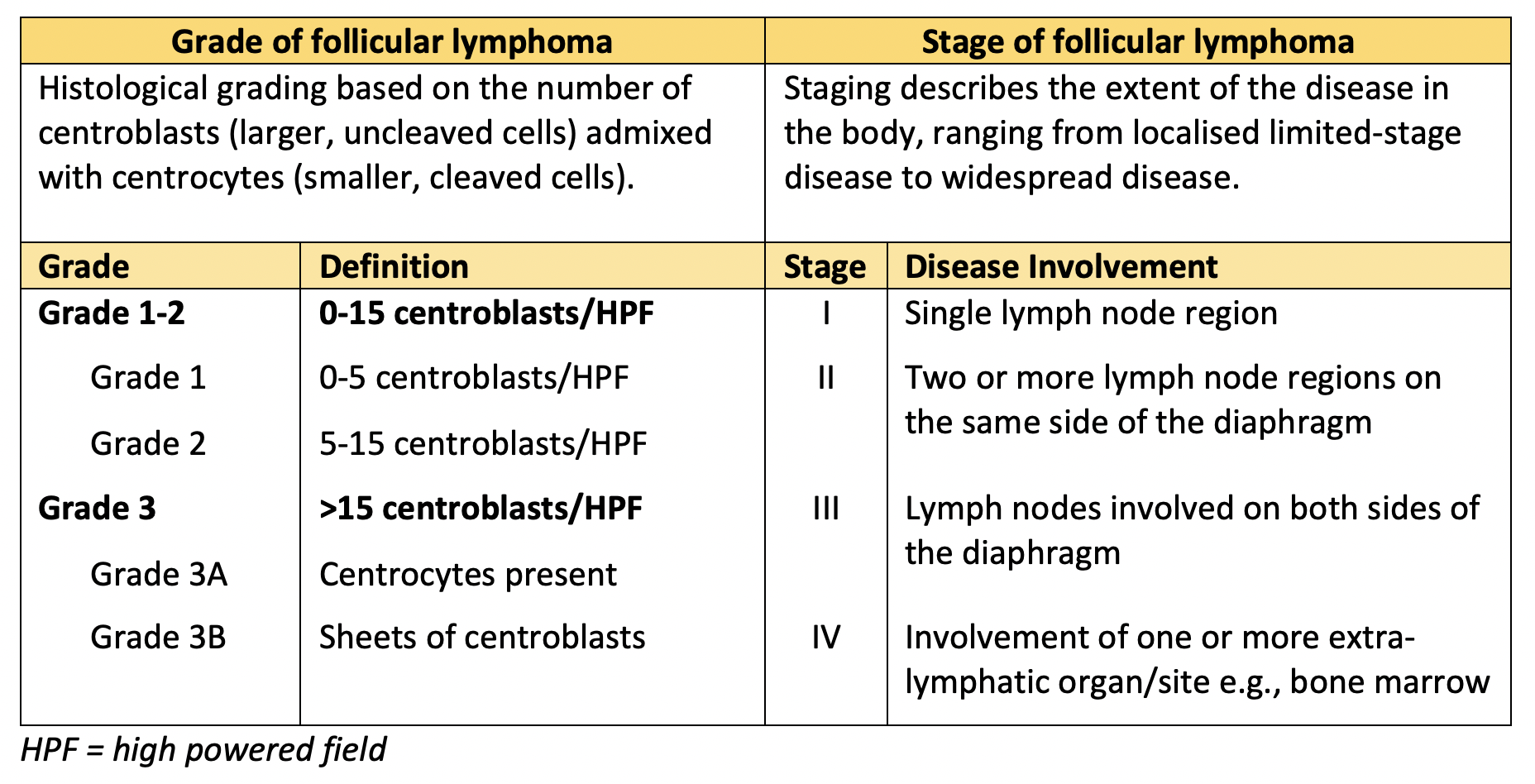
A biopsy diagnosed grade 3A follicular lymphoma with a proliferative index (Ki-67) of 40%. A PET scan showed disease limited to the abdomen with enlarged, FDG-avid nodes in the retroperitoneum, right retrocrural and bilateral groin regions, in addition to the left mesenteric mass. This gave a stage of IIAX (A = asymptomatic, X = bulk).
Table 1 describes how grading and staging are assessed in follicular lymphoma.
He completed six cycles of G-CHOP chemotherapy (obinutuzumab, cyclophosphamide, doxorubicin, vincristine, prednisolone) that were well tolerated without any adverse events and was delivered as an outpatient. An end-of-treatment PET scan showed the mass had decreased from 13.5 cm to 7.9 cm with moderate metabolic reduction consistent with a partial metabolic response. The other avid lymph nodes seen on the initial PET scan had resolved.
Following the completion of induction chemoimmunotherapy, the patient commenced maintenance obinutuzumab. Obinutuzumab 1000 mg was given every two months with a plan for two years of treatment.
A year later, after 12 months of maintenance therapy, the patient was admitted to hospital with atypical chest pain and a CT scan identified mediastinal as well as progressive intra-abdominal lymphadenopathy with increase in size of the mesenteric mass to 12.9 cm.
A biopsy confirmed grade 2/3A relapsed follicular lymphoma.
Salvage chemotherapy with R-DHAC (rituximab, dexamethasone, cytarabine, carboplatin) was commenced.
After one cycle, a PET scan showed significant reduction in activity of lymphadenopathy in keeping with a partial metabolic response. Two cycles of R-DHAC chemotherapy were completed with a plan to proceed to an autologous stem cell transplant.
However, progressive disease occurred prior to the planned autologous stem cell transplant and the patient was therefore enrolled onto a clinical trial involving a novel phosphatidylinositol-3-kinase (PI3K) delta inhibitor.
A biopsy was performed before trial entry and confirmed grade 1-2 follicular lymphoma with focal areas of grade 3A disease. After two cycles of the PI3K delta inhibitor, a restaging CT and PET scan showed a response with a reduction in his mesenteric mass from 12.8 cm to 10.9 cm.
The patient is currently well and has not developed any adverse events from his treatment.
Bispecific antibodies
In B-cell malignancies, immune cellular therapies attempt to engage the patient’s T cells to kill malignant B cells. Bispecific antibodies (bsAbs) are molecules designed to recognise two different antigens with the majority facilitating engagement and activation of T-cells resulting in cellular-dependent cytotoxicity against tumour cells2.
Blinatumomab, a CD3/CD19 bsAb, is currently the only bsAb approved in Australia for B-cell malignancies. However, its use is currently limited to the treatment of relapsed/refractory (R/R) B-cell acute lymphoblastic leukaemia and it is less effective in non-Hodgkin lymphomas such as follicular lymphoma.
Recently, a range of CD3/CD20 bsAbs with a much longer half-life than blinatumomab has been developed and the bsAbs are in early phase clinical trials including patients with R/R follicular lymphoma. Data has been presented for a number of CD3/CD20 bsAbs including mosunetuzumab, glofitamab, epcoritamab and odronextamab.
The CD3/CD20 bsAb mosunetuzumab has been assessed in patients with R/R follicular lymphoma in the phase I/Ib trial G029781 (NCT02500407). Data presented included 62 patients with follicular lymphoma treated with mosunetuzumab after at least two prior systemic therapies3. The overall response rate was 67.7% with 51.6% of patients achieving a complete remission3. The median duration of response was 20.4 months (95% CI: 9.4-22.7)3.
Another CD3/CD20 bsAb, odronextamab, has been evaluated in patients with R/R follicular lymphoma in a phase I study (NCT02290951). In 37 patients with grade 1-3a FL, odronextamab, at a dose of ?5 mg (n=28), led to an overall response rate of 92.9% with a complete remission rate of 75%4. The median duration of response was 7.7 months4.
Complications of bsAbs include the effect of potent T-cell activation, including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), tumour flare and tumour lysis syndrome. Cytokine release syndrome varies in severity ranging from a fever alone to hypoxia requiring positive pressure support and hypotension requiring vasopressors. In a phase I study with glofitamab, the rate of cytokine release syndrome was 55% but grade 3 or 4 cytokine release syndrome was seen in only 1.7% of patients5. ICANS is uncommon when step-up dosing is performed with a phase I/Ib trial of mosunetuzumab in R/R NHL reporting only 1.4% of patients experienced grade 1 or 2 ICANS-like neurological adverse events6. Methods to mitigate these risks include a step-up dosing schedule and pre-treatment with an anti-CD20 antibody.
Treatment of cytokine release syndrome includes the use of tocilizumab, an anti-IL6 receptor antibody, while ICANS is treated with high dose corticosteroids.
Chimeric T-cell antigen receptor therapy
Chimeric antigen receptor (CAR)-T cell therapy has been shown to be effective in patients with multiply relapsed follicular lymphoma7–9.
CAR-T cell therapy is a form of cellular immune therapy where the patient’s T cells are harvested, then engineered to express a chimeric antigen receptor against CD19, the target B-cell antigen7. The T cells are genetically modified ex-vivo, expanded in number and are then reinfused into the patient. Various CAR-T constructs exist with differences in their structure, but head-to-head comparisons have not been performed.
Evidence for the use of CAR-T cells in follicular lymphoma includes single-arm prospective trials7,10. Recently presented data includes results from CD19-directed CAR-T agents axicabtagene ciloleucel (axi-cel) in the single-arm ZUMA-5 trial as well as the Phase 2 ELARA trial with tisagenlecleucel (tisa-cel)8,9.
In the ZUMA-5 trial, there were 124 patients with R/R follicular lymphoma who were eligible if they had follicular lymphoma (grades 1-3a) with two or more prior lines of therapy, including an anti-CD20 monoclonal antibody plus an alkylating agent, with an Eastern Cooperative Oncology Group (ECOG) score of 0 or 1. Patients received cyclophosphamide/fludarabine conditioning followed by a single infusion of axi-cel at 2 x 106 CAR-T cells per kilogram.
Data among efficacy-evaluable patients was available in 84 patients with follicular lymphoma. The overall response rate was 85% with a complete remission rate of 76%9. At a median follow-up of 17.5 months, the 12-month estimated duration of response was 72% (95% CI, 61-80%) and progression-free survival was 74% (95% CI, 63-82%)9.
Grade ?3 cytokine release syndrome occurred in 6% of patients with follicular lymphoma and grade ?3 neurologic events occurred in 15%9. Three patients died, with one related to axi-cel where multisystem organ failure occurred in the context of cytokine release syndrome9. Similar response rates and toxicity were seen in the patients treated on the ELARA trial with tisa-cel.
Apart from the risk of potentially life-threatening cytokine release syndrome and neurologic events, other adverse events including prolonged cytopenias, serious infections and prolonged hypogammaglobulinemia may occur. These possible adverse events will be important considerations if CAR-T therapy becomes available in routine practice.
While this data is promising for the efficacy of CAR-T cell therapy in R/R follicular lymphoma, the treatment comes with the risk of significant toxicity and the manufacturing process is expensive and time consuming.
Additionally, real-world outcomes with CAR-T cell therapy may not achieve the response rates seen in clinical trials as patients with poor performance status, significant co-morbidities and rapidly progressive disease are generally excluded from trial. Manufacturing issues may also occur, such as poor T-cell growth or patients having a low lymphocyte count insufficient for successful apheresis of T-cells.
Phosphatidylinositol-3 kinase inhibitors
The phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT) pathway is frequently dysregulated in NHL and promotes cell proliferation while reducing apoptosis11. Cell membrane growth factor receptors activate PI3K, which then phosphorylates the membrane phospholipid phosphatidylinositol11. This leads to AKT activation and downstream signalling that promotes both cell growth and resistance to apoptosis11.
Inhibitors of the PI3K/AKT pathway have demonstrated efficacy in multiply relapsed follicular lymphoma but significant safety issues have been observed. In a phase 2 study, the oral inhibitor of PI3K delta, idelalisib, was assessed in 125 patients with relapsed indolent NHL, 72 of whom had follicular lymphoma12. The overall response rate was 57%, with 6% achieving a complete remission12. The median progression free survival was 11 months12.
Significant safety concerns with idelalisib include fatal or serious diarrhoea, hepatotoxicity, intestinal perforation, colitis and pneumonitis. Patients should be closely monitored for liver function derangement and appropriately dose reduced as per the product information.
Opportunistic infections are increased with idelalisib and include Pneumocystis jirovecii pneumonia and CMV reactivation. Pneumocystis jirovecii pneumonia prophylaxis is recommended and CMV prophylaxis may be considered. Real world data demonstrates that follicular lymphoma patients treated with idelalisib in routine clinical practice, compared with those on clinical trial, are older, have more co-morbidities and are at higher risk of fatal infections13.
Novel PI3K inhibitors have been developed with the hope that increased specificity for the PI3K? isoform will improve safety. There are four class I PI3K catalytic isoforms: p110?, p110?, p110? and p110?[14]. PI3K? and AKT inhibitors are being studied in breast and prostate cancer whereas PI3K? inhibitors are being used in NHL since PI3K? and PI3K? are highly expressed in white blood cells.
Recent data from the phase 3 CHRONOS-3 trial assessed the intravenous PI3K inhibitor copanlisib, with predominant activity against PI3K? and PI3K? isoforms, in patients with follicular lymphoma and chronic lymphocytic leukaemia15. Copanlisib was combined with rituximab in 307 patients and was compared with placebo plus rituximab in 151 patients. The overall response rate was higher in the copanlisib-rituximab arm (81% vs 48%) with a median progression-free survival of 21.5 months compared with 13.8 months in the rituximab-alone group (95% CI 0.39-0.69; p<0.0001)15. The complete remission rate was 34% in the copanlisib-rituximab group compared with 15% in the rituximab-alone group15.
Adverse events in the CHRONOS-3 trial were higher in the copanlisib-rituximab group. Grade 3 or 4 pneumonitis occurred in 3% of patients in the copanlisib-rituximab group and one patient died from drug-related pneumonitis15. Grade 3 diarrhoea occurred in 5% of patients treated with copanlisib-rituximab15.
Although hyperglycaemia and hypertension were common with copanlisib, these events were infusion related and were manageable with glucose-lowering agents after infusion and antihypertensives. Despite the fact copanlisib-rituximab was deemed safe, there was a higher rate of discontinuation compared with rituximab because of treatment-emergent adverse events (31% vs 8%)15.
Conclusion
The treatment of relapsed or refractory follicular lymphoma is challenging in patients who progress early after initial chemoimmunotherapy or in those who have been treated with multiple prior lines of therapy.
Patients may become refractory to chemotherapy, and agents with novel mechanisms of action are needed in this setting. Despite these new agents, there remains a role for radiotherapy, and autologous and allogeneic stem cell transplant in R/R follicular lymphoma but a discussion regarding these therapies is beyond the scope of this article.
Cellular therapies such as bispecific antibodies and CAR-T cell therapy offer promising response rates. However, an understanding of the management of their unique toxicities, such as cytokine release syndrome and neurotoxicity, is important.
New therapies with reduced toxicity are important and agents such as novel PI3K inhibitors may offer a balance between efficacy and adverse events. Prophylaxis for Pneumocystis jirovecii pneumonia, CMV monitoring and dose adjustments with hepatoxicity are essential especially in at-risk older patients with co-morbidities and those with a history of recent infections.
This article has reviewed only three of these new non-chemotherapy therapies but there a number of other exciting agents demonstrating efficacy in follicular lymphoma. These agents are being used in both the treatment-naïve and R/R setting and include lenalidomide, an immunomodulatory imide drug (IMiD); ibrutinib, a Bruton tyrosine kinase inhibitor; tazemetostat, an EZH2 inhibitor; and checkpoint inhibitors such as nivolumab.
As further evidence from clinical trials becomes available, the hope is that these therapies will move into daily clinical practice and may one day lead to an effective chemotherapy-free approach to relapsed follicular lymphoma.
Dr Ross Salvaris is a haematologist with an interest in lymphoma currently working in the Clinical Trials Centre at Monash Health.
References
- Tan, D., Horning, S. J., Hoppe, R. T., Levy, R., Rosenberg, S. A., Sigal, B. M., et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood 122, 981–987 (2013). DOI: 10.1182/blood-2013-03-491514.
- Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. W. H. I. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 18, 585–608 (2019). DOI: 10.1038/s41573-019-0028-1.
- Assouline, S. E., Kim, W. S., Sehn, L. H., Schuster, S. J., Cheah, C. Y., Nastoupil, L. J., et al. Mosunetuzumab Shows Promising Efficacy in Patients with Multiply Relapsed Follicular Lymphoma: Updated Clinical Experience from a Phase I Dose-Escalation Trial. Blood 136, 42–44 (2020). DOI: 10.1182/blood-2020-135839.
- Bannerji, R., Allan, J. N., Arnason, J. E., Brown, J. R., Advani, R., Ansell, S. M., et al. Odronextamab (REGN1979), a Human CD20 x CD3 Bispecific Antibody, Induces Durable, Complete Responses in Patients with Highly Refractory B-Cell Non-Hodgkin Lymphoma, Including Patients Refractory to CAR T Therapy. Blood 136, 42–43 (2020). DOI: 10.1182/blood-2020-136659.
- Dickinson, M., Morschhauser, F., Iacoboni, G., Carlo-Stella, C., Offner, F. C., Sureda Balari, A., et al. CD20-TCB in Relapsed or Refractory Non-Hodgkin Lymphoma: Durable Complete Responses and Manageable Safety Observed at Clinically Relevant Doses in Phase I Dose Escalation. EHA Library S241 https://library.ehaweb.org/eha/2020/eha25th/293690/michael.j.dickinson.cd20?tcb.in.relapsed.or.refractory.nonhodgkin.lymphoma.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1 (2020).
- Schuster, S. J., Bartlett, N. L., Assouline, S., Yoon, S. S., Bosch, F., Sehn, L. H., et al. Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, and Is Active in Treatment through Multiple Lines. Blood 134, 6 (2019). DOI: 10.1182/blood-2019-123742.
- Schuster, S. J., Svoboda, J., Chong, E. A., Nasta, S. D., Mato, A. R., Anak, Ö., et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 377, 2545–2554 (2017). DOI: 10.1056/nejmoa1708566.
- Fowler, N. H., Dickinson, M., Dreyling, M., Martinez-Lopez, J., Kolstad, A., Butler, J. P., et al. Efficacy and Safety of Tisagenlecleucel in Adult Patients with Relapsed/Refractory Follicular Lymphoma: Interim Analysis of the Phase 2 Elara Trial. Blood 136, 1–3 (2020). DOI: 10.1182/blood-2020-138983.
- Jacobson, C., Chavez, J. C., Sehgal, A. R., William, B. M., Munoz, J., Salles, G., et al. Primary Analysis of Zuma-5: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory (R/R) Indolent Non-Hodgkin Lymphoma (iNHL). Blood 136, 40–41 (2020). DOI: 10.1182/blood-2020-136834.
- Hirayama, A. V., Gauthier, J., Hay, K. A., Voutsinas, J. M., Wu, Q., Pender, B. S., et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 134, 636–640 (2019). DOI: 10.1182/blood.2019000905.
- Schatz, J. H. Targeting the PI3K/AKT/mTOR pathway in non-Hodgkin’s lymphoma: Results, biology, and development strategies. Curr. Oncol. Rep. 13, 398–406 (2011). DOI: 10.1007/s11912-011-0187-7.
- Gopal, A. K., Kahl, B. S., de Vos, S., Wagner-Johnston, N. D., Schuster, S. J., Jurczak, W. J., et al. PI3K? Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N. Engl. J. Med. 370, 1008–1018 (2014). DOI: 10.1056/nejmoa1314583.
- Bird, S. T., Tian, F., Flowers, N., Przepiorka, D., Wang, R., Jung, T. H., et al. Idelalisib for Treatment of Relapsed Follicular Lymphoma and Chronic Lymphocytic Leukemia: A Comparison of Treatment Outcomes in Clinical Trial Participants vs Medicare Beneficiaries. JAMA Oncol. 6, 248–254 (2020). DOI: 10.1001/jamaoncol.2019.3994.
- Bilanges, B., Posor, Y. & Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle trafficking. Nature Reviews Molecular Cell Biology vol. 20 515–534 (2019). DOI: 10.1038/s41580-019-0129-z.
- Matasar, M. J., Capra, M., Özcan, M., Lv, F., Li, W., Yañez, E., et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma. Lancet Oncol. 2045, (2021). DOI: 10.1016/S1470-2045(21)00145-5.