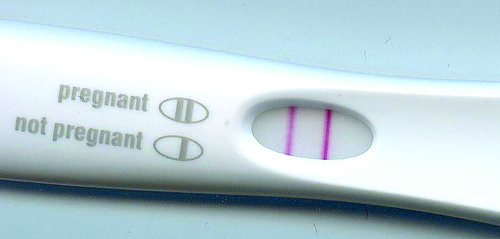
A significant number of commercially available DIY pregnancy testing have failed to meet sensitivity standards
A TGA review of self-testing pregnancy kits found a significant number of commercially available products failed to meet sensitivity standards and had to be withdrawn from the market.
The review of all human chorionic gonadotropin (hCG) kits was prompted by reports of false-negative findings by some kits which lacked sufficient sensitivity to detect low levels of the hCG hormone.
Of the 38 kits tested, five failed to work reliably or accurately and a further nine kits were voluntarily withdrawn from sale ahead of the review.
“Samples of the remaining 27 devices were acquired and tested for whether they recognised only hCG (not other hormones), how sensitive they were to hCG and if the labelling was correct,” the TGA said in a statement.
“A total of 22 of 27 (81%) test kits sampled passed testing and were shown to work reliably. The five devices that failed have been subjected to a range of regulatory actions.”
The TGA said it was considering “further regulatory action” against one supplier: Thermo Fisher Scientific Australia, which produces the QuickVue One-Step hCG kit.
Another product, First Response Digital (Church and Dwight) could not be tested because too many of the devices malfunctioned.
The company subsequently withdrew the kit from sale and an investigation revealed a manufacturing flaw, which has since been corrected.
All devices remaining on the market in Australia had been shown to work reliably and accurately, the TGA said.