This condition is generally benign, but a minority can have life-threatening complications.
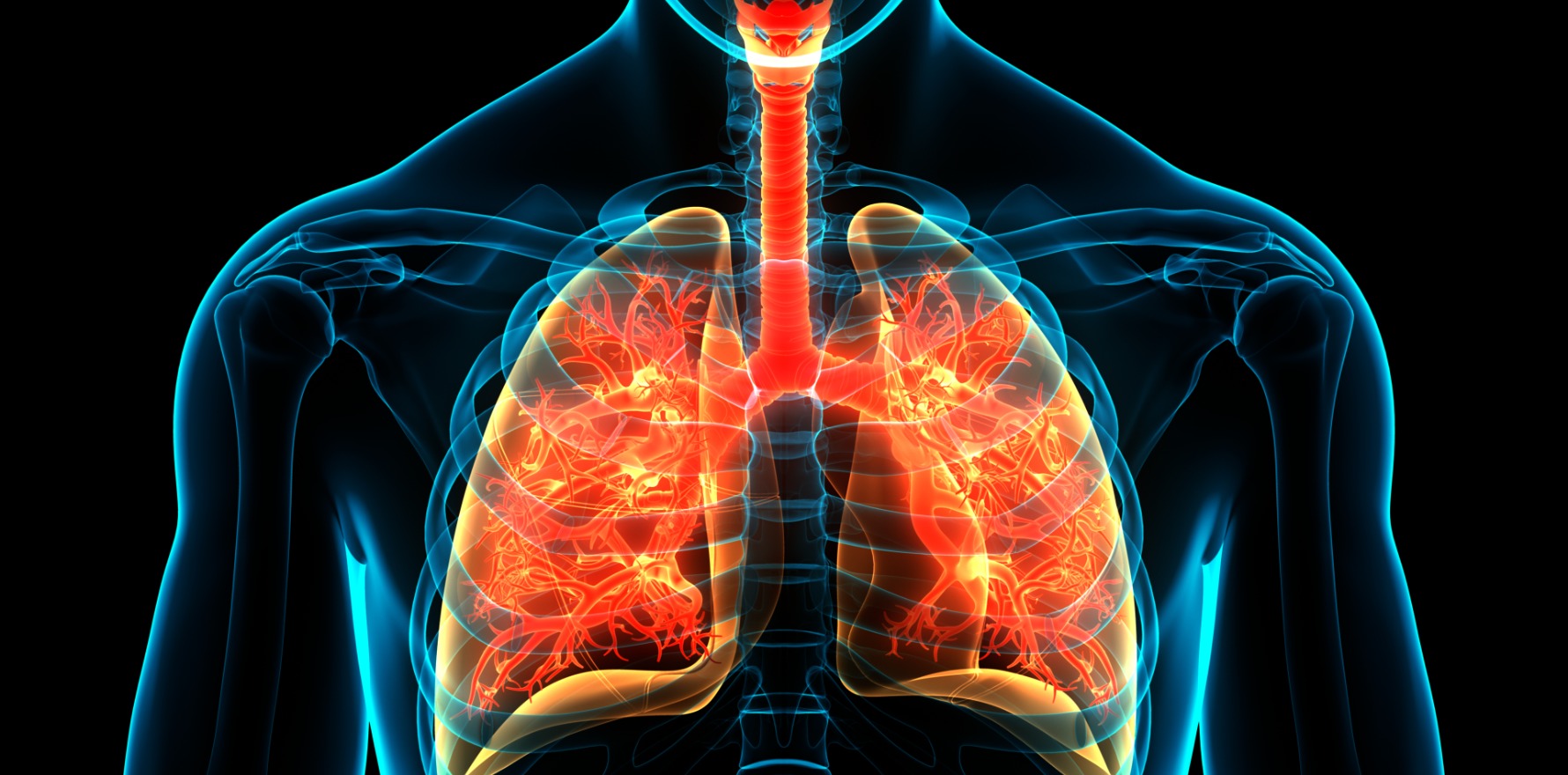
Sarcoidosis is a systemic disorder with a diverse set of clinical manifestations that usually present in general practice.
Most patients are not diagnosed on their first presentation, with one study demonstrating that as many as 86.5% are not diagnosed until subsequent visits.1
It most typically presents in patients between the ages of 20 to 60 years, the prevalence being estimated at 4.4 to 6.3 per 100,000 in Australia.2
The hallmark of sarcoidosis is the histological presence of non-caseating granulomas in affected organs, as a result of cell-mediated immune responses against as yet unidentified antigens. Its aetiology remains enigmatic, the evidence suggesting a role of exposure of genetically susceptible individuals to environmental agents.
More details of the background pathogenesis and treatment beyond this article are available in a summary of the Thoracic Society of Australia and New Zealand’s state of the art review of sarcoidosis.3
The diagnosis of sarcoidosis presents a challenge for general practitioners – the clinical presentation can be non-specific, and include lethargy, fever, particularly night sweats, and general malaise. These symptoms should raise the possibility of sarcoidosis if other more common causes of fatigue have been excluded (e.g. hypothyroidism, iron deficiency, neoplasia, and auto-immune disorders).
More specific symptoms range from non-productive cough and breathlessness, to ocular, joint, skin, or cardiac manifestations.4 Notably, sarcoidosis most commonly involves the lungs, but a significant proportion of patients present with extrathoracic manifestations.3
Ocular involvement is the presenting symptom of sarcoidosis in up to 5% of patients, in addition to being a frequent (20-50%) and early feature of the disease.4,5 It may be suggested by patients presenting with pain, dry eyes, blurred vision, photophobia, or redness.
A significant minority of patients (10-15%) have an associated arthropathy6 with acute sarcoid arthritis presenting most often as part of Löfgren’s syndrome. This arthritis commonly involves the ankles (>90%).7
Cardiac involvement is uncommon (See table 1), but has important repercussions for patients, with risk of dysrhythmias and death. Cardiac sarcoidosis may present with chest pain, palpitations, breathlessness, and unexplained syncope/presyncope due to dysrhythmias. An ECG is appropriate initially in all patients, mainly to detect intraventricular and AV conduction defects.3,4,8
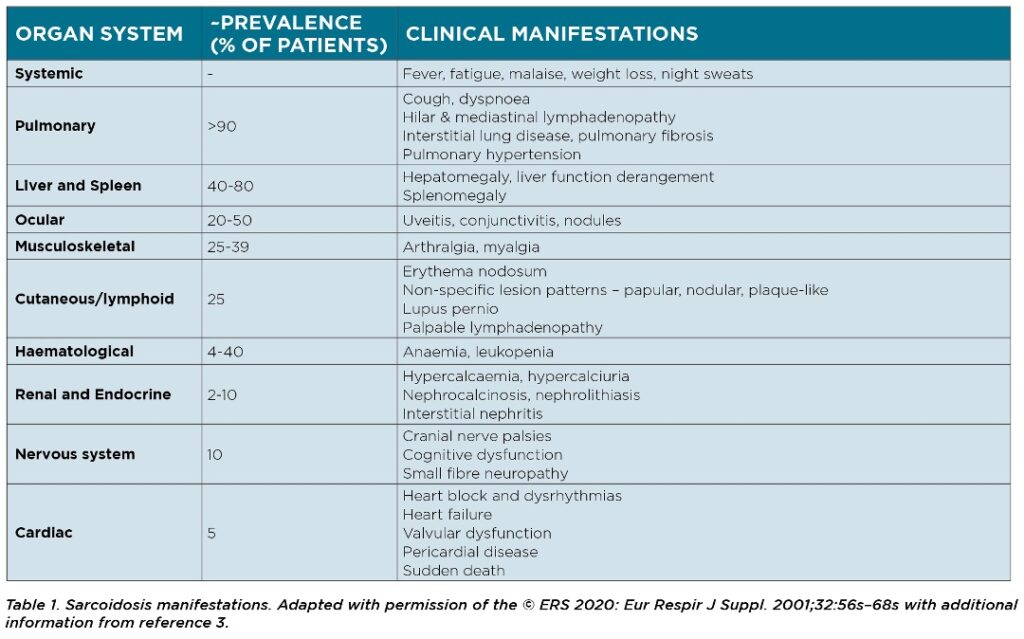
Löfgren’s syndrome is a distinct sub-set of sarcoidosis9 which presents in a variable proportion of sarcoidosis patients, and is characterised by bilateral hilar lymphadenopathy on chest X-ray, erythema nodosum, fever, and migratory polyarthritis.
A differential diagnosis of sarcoidosis may include mycobacterial and fungal infections, and other granulomatous lung diseases such as hypersensitivity pneumonitis. Naturally, the presence of prominent systemic symptoms raises the level of concern for malignancy or infection.
A number of investigations are key to diagnosis of sarcoidosis whilst eliminating differential diagnoses, many of which are easily performed in the general practice setting.
Generally, full blood count, liver function tests, electrolytes and serum angiotensin converting enzyme levels (ACE) should be checked.10 It is essential to check for hypercalcaemia (occurring in 10-20% of patients11) and hypercalciuria.3 Serum ACE is elevated in 60-80% of patients with sarcoidosis and may reflect total granuloma burden.12 Although it is not specific, it can indicate sarcoidosis as a possible diagnosis.3
Imaging is also an important diagnostic modality. A chest X-ray is the first line investigation, and bilateral hilar lymphadenopathy is often an incidental finding on chest X-ray in otherwise asymptomatic persons who have had the investigation for other purposes.3
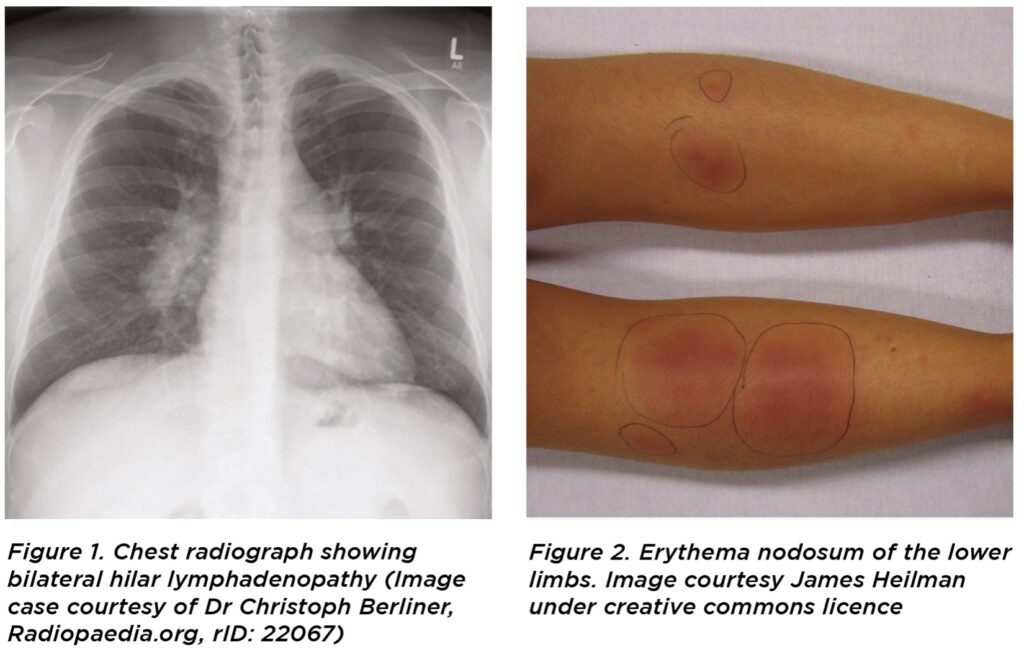
Chest CT is a particularly useful modality and can imply the diagnosis if showing classic parenchymal findings (nodules clustered along bronchovascular bundles, interlobular septa and subpleurally, with hilar and mediastinal lymphadenopathy).3
Ultimately, a biopsy indicating a non-caseating granulomata provides the best supportive evidence of the diagnosis. Biopsies should be performed on the most readily accessible affected organ, such as skin or peripheral lymph nodes, and lip and conjunctival samples can be obtained also. As the lungs and intrathoracic lymph nodes are most commonly affected, bronchoscopy with endobronchial, transbronchial biopsy or needle aspiration of lymph nodes is often performed.3,13
Pulmonary function tests are also important to assess respiratory impairment and to monitor sarcoidosis, with two-thirds of patients developing airway obstruction.3 Other investigations may be guided by patient symptoms and signs.
Complications of sarcoidosis are uncommon, but may include development of pulmonary hypertension from lung fibrosis, airway obstruction, fungal infections, hypercalcaemia, and increased risk of venous thromboembolic disease (VTE).14 It is particularly important to recognise uveitis and cardiac involvement, and regular reviews are recommended.3
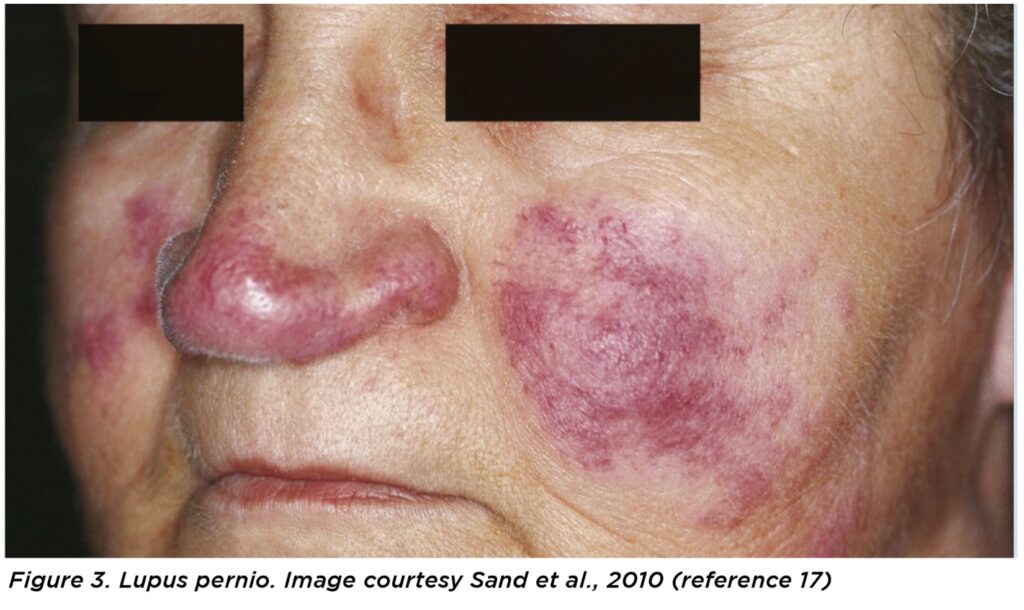
Management of sarcoidosis is guided by patient symptoms and disease progression, and there are few studies to guide therapy. Generally, asymptomatic patients with minimal physiological impairment do not require treatment, and have an excellent prognosis. Over 70% of patients eventually show no evidence of active disease.3
For those that require therapy, systemic corticosteroids remain the first-line option.3, 15 Other therapies with varying levels of evidence include immunosuppressive agents, such as methotrexate, azathioprine, hydroxychloroquine, and monoclonal antibodies e.g. TNF inhibitors. These agents carry their own risks.3 Regular monitoring of all patients for the development of disease progression and complications is recommended; at least annually or more frequently in active disease (particularly electrocardiography for evidence of conduction abnormalities, and ophthalmological evaluation).
In summary, sarcoidosis is a complex systemic disease with the potential to elude diagnosis in general practice.
A number of investigative findings may suggest a diagnosis of sarcoidosis, and ultimately histological presence of non-caseating granulomas on biopsy is diagnostic.
Treatment is not always warranted, and should be guided by patient factors on a case-by-case basis.
Katrin Mannes and Professor Paul S. Thomas are from the Department of Respiratory Medicine, Prince of Wales Hospital, Sydney and Prince of Wales Clinical School, Faculty of Medicine, UNSW Sydney. Professor Thomas is also from the Mechanisms of Disease and Translational Research Medicine at UNSW
References
- Judson M, Thompson B, Rabin D, et al. The Diagnostic Pathway to Sarcoidosis. Chest. 2003;123(2):406-412.
- Gillman A, Steinfort C. Sarcoidosis in Australia. Internal Medicine Journal. 2007;37(6):356-359.
- Ahmadzai H, Huang S, Steinfort C, et al. Sarcoidosis: a state of the art review from the Thoracic Society of Australia and New Zealand. MJA. 2018;208(11):499-504.
- Soto-Gomez N, Peters J, Nambia, A. Diagnosis and Management of Sarcoidosis. American Family Physician. 2016;93(10):840-850.
- Jamilloux Y, Kodjikian L, Broussolle C, Sève P. Sarcoidosis and uveitis. Autoimmunity Reviews. 2014;13(8):840-849.
- Ungprasert P, Crowson C, Matteson E. Clinical Characteristics of Sarcoid Arthropathy: A Population-Based Study. Arthritis Care & Research. 2016;68(5):695-699.
- Visser H, Vos K, Zanelli E, et al. Sarcoid arthritis: clinical characteristics, diagnostic aspects, and risk factors. Annals of the Rheumatic Diseases. 2002;61(6):499-504.
- Birnie D, Sauer W, Bogun F, et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated With Cardiac Sarcoidosis. Heart Rhythm. 2014;11(7):1304-1323.
- Löfgren S, Lundback H. The bilateral hilar lymphoma syndrome; a study of the relation to tuberculosis and sarcoidosis in 212 cases. Acta Med Scand 1952;142:265–273.
- Dempsey O, Paterson E, Kerr K, Denison A. Sarcoidosis. BMJ. 2009;339:b3206-b3206.
- Rao D, Dellaripa P. Extrapulmonary Manifestations of Sarcoidosis. Rheumatic Disease Clinics of North America. 2013;39(2):277-297.
- Iannuzzi M, Rybicki B, Teirstein A. Sarcoidosis. NEJM. 2007;357(21):2153-2165.
- Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011; 183: 573-581.
- Ungprasert P, Crowson C, Matteson E. Association of sarcoidosis with Increased risk of VTE: A population-based study, 1976 to 2013. Chest. 2017;151(2):425-430.
- Iannuzzi M, Fontana J. Sarcoidosis. JAMA. 2011;305(4):391.
- Costabel U. Sarcoidosis: clinical update. Eur Respir J Suppl 2001;32:59s.
- Sand M, Sand D, Thrandorf C, et al. Cutaneous lesions of the nose. Head & Face Medicine. 2010;6(1).