And the Indian strain of SARS-CoV-2 has been labelled a 'variant of concern'.
Welcome to The Medical Republic’s COVID Catch-Up.
It’s the day’s COVID-19 news in one convenient post. Email bianca@biancanogrady.com with any tips, comments or feedback.
12 May
- The US FDA grants emergency use authorisation to the Pfizer/BioNTech vaccine for 12-15-year-olds
- Indian strain of SARS-CoV-2 labelled ‘variant of concern‘ due to increased transmissibility.
- Victoria issues list of venues visited by infectious case, but no new cases reported.
- Even mild, non-hospitalised cases of COVID-19 are associated with more subsequent GP visits, breathlessness and thromboembolism.
- US CDC gives advice on how to talk to friends and family about COVID-19 vaccines.
- New infections decreased globally in the past week, but are still rising in India and neighbouring nations.
- Latest COVID-19 infection numbers from around Australia.
The Pfizer/BioNTech vaccine has been approved in the United States for use in children aged 12 years and above.
The US Food and Drug Administration has expanded the emergency use authorisation of the mRNA vaccine to include adolescents, based on evidence that the known and potential benefits of the vaccine in younger individuals outweighed the known and potential risks.
That evidence included data from studies involving 2260 individuals aged 12 to 15 years, who participated in randomised, placebo-controlled trials of the vaccine in the US, and who were followed for at least two months after their second dose.
These studies found the immune response to the vaccine in the younger age group was equivalent to that seen in adults, and the vaccine was 100% effective at preventing infection.
The most common side effects were pain at injection site, fatigue, headache, chills, muscle aches, fever and joint pain, and more of these side effects were reported after the second dose than the first dose.
The FDA announcement also reiterated that the vaccine should not be given to anyone with a history of severe allergic reaction or anaphylaxis to any of the vaccine components.
The World Health Organisation has labelled the new strain of SARS-CoV-2 devastating India as a ‘variant of concern’, highlighting B.1.617’s increased transmissibility and reduced susceptibility to treatments such as the antibody bamlanivimab and to neutralising antibodies.
The strain, which was first reported in India in October 2020, consists of three sub-lineages, based on different mutations in the virus’ spike protein.
WHO said that the resurgence of COVID-19 in India in recent months was likely a combination of the increased transmissibility of this strain, reduced adherence to and use of public health measures, and mass gatherings that had been held for political and religious events.
However it’s not yet clear whether the strain is still susceptible to existing COVID-19 vaccines. Preliminary laboratory studies – as yet unpublished – suggest the Moderna and Pfizer/BioNTech vaccines may have reduced neutralising effects against B.1.617, but it’s not known how this will translate in a real-world setting.
The Victorian health department has issued a list of venues visited by an individual who tested positive after returning to the state, despite previously undergoing two weeks in hotel quarantine in South Australia.
They include Southern Cross and Craigieburn railway stations on 7 May, several venues in Epping on the 6 and 8 May, and city metro train services on the 7 May. So far no new cases have been reported, and no restrictions have been imposed.
Meanwhile, continuing investigations in NSW have still failed to identify the infectious case linking a Sydney man with a returned traveller in hotel quarantine.
Mild COVID-19 infections that don’t require hospitalisation are still associated with an increased risk of subsequent thromboembolism, breathlessness, and more GP visits, a study has found.
A paper published in The Lancet Infectious Diseases reports the results of a population-based cohort study comparing six-month outcomes in nearly 9000 individuals diagnosed with COVID-19 in Denmark but not admitted to hospital, with those of more than 80,000 people without COVID-19 and 1310 people admitted to hospital with COVID-19.
Those who tested positive but were not hospitalised had a two-fold greater odds of being diagnosed with dyspnoea for the first time, 77% higher odds of thromboembolism, and 23% higher odds of starting bronchodilators in the six-month follow-up period than those without COVID-19. They also showed an 18% increase in the likelihood of visiting their GP.
“Because most SARS-CoV-2-infected individuals are managed in the community, it is of major public health importance to better understand the risk of delayed effects in non-hospitalised individuals,” the authors wrote.
Reflecting the significant challenge of rolling out the biggest-ever vaccine campaign at a time when vaccine hesitancy is at an all-time high, the US Centers for Disease Control has published some brief advice on how to talk about COVID-19 vaccines with friends and family, including video guidance.
A glimmer of good news in the global infection tally: new cases have decreased slightly in the past week for the first time in 10 weeks, according to the latest update from the World Health Organisation.
Just over 5.5 million new cases were reported in the past week, around half of which occurred in India. More than 90,000 people died from COVID-19 in the past week, one-third of whom were also in India.
While infections continue to increase in the stricken nation, the rate does appear to have slowed from the previous week, with only a 5% increase in new cases. However there are concerns that India’s outbreak may be spreading to nearby nations such as Nepal, which is experiencing a massive increase in infections.
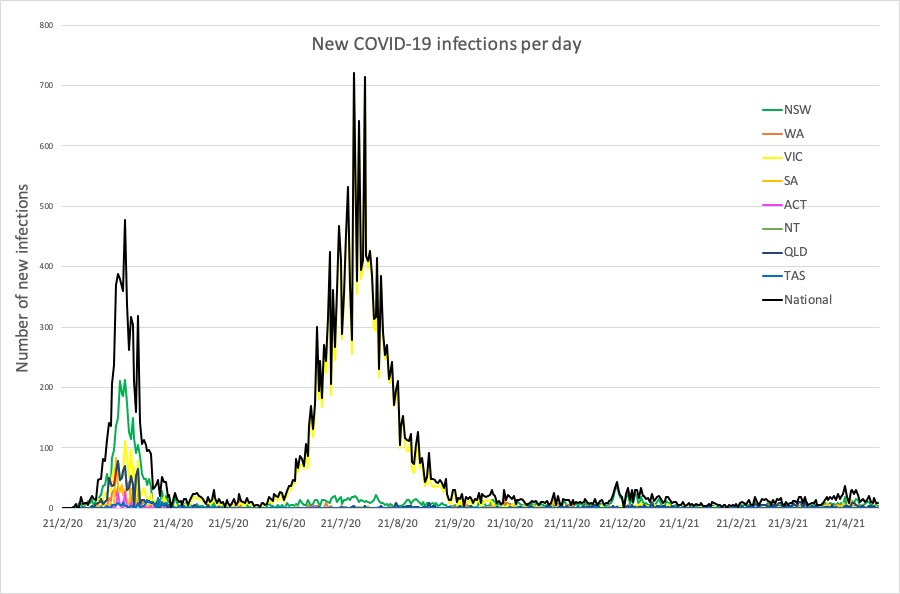
Here are the latest COVID-19 infection numbers – and new cases in the past 24 hours – from around Australia, to 9pm Tuesday:
National – 29,939 with 910 deaths
ACT – 124 (0)
NSW – 5542 (4)
NT – 167 (0)
QLD – 1581 (0)
SA – 740 (0)
TAS – 234 (0)
VIC – 20,536 (1)
WA – 1015 (2)