And Janssen's single-dose vaccine gets approval from WHO.
Welcome to The Medical Republic’s COVID Catch-Up.
It’s the day’s COVID-19 news in one convenient post. Email bianca@biancanogrady.com with any tips, comments or feedback.
15 March
- Watch for vaccine-associated lymphadenopathy when screening women for breast cancer.
- WHO approves Janssen single-dose vaccine for emergency use.
- AstraZeneca says no evidence of clot risk from its COVID-19 vaccine
- TGA approves higher storage temperatures for Pfizer/BioNTech vaccine.
- Brisbane healthcare worker and Sydney quarantine hotel guard test positive for SARS-CoV-2.
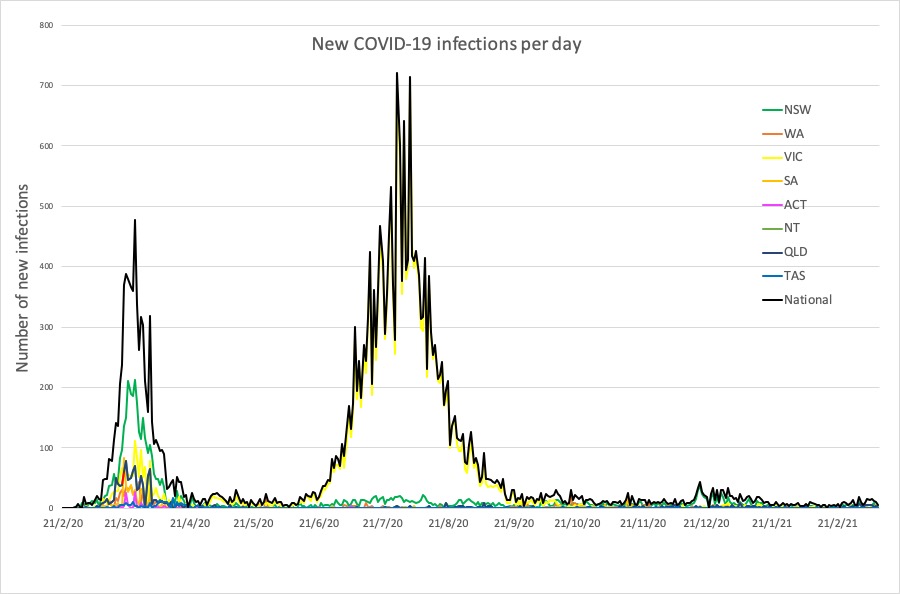
- Latest COVID-19 infection numbers from around Australia.
Women undergoing breast screening should be asked whether they have recently received the COVID-19 vaccine due to the risk of lymphadenopathy associated with the vaccine, says the UK’s Drug Safety Research Unit.
Around 11%-16% of people who receive the MRNA-based COVID-19 vaccines experience lymph node swelling, most commonly in the armpit closest to the vaccination site.
“It is therefore expected that, following recent COVID-19 vaccination, a number of women will present with newly palpable axillary adenopathy or that these will be detected during routine breast screening,” unit staff wrote in a statement.
In the US, the advice has been to either undergo breast screening before vaccination or wait until 4-6 weeks after vaccination.
AstraZeneca has reassured the world that there is no evidence of increased risk of pulmonary embolism or deep vein thrombosis associated with its COVID-19 vaccine, despite being used in more than 17 million people so far.
In a statement, the manufacturer said it had reviewed all the available safety data, and found no safety signals for any particular batch, age group, gender or country of use.
Commenting on the 15 DVT and 22 PE events reported so far, the company commented that this number was lower than would be expected to occur in the general population.
The single-dose Janssen COVID-19 vaccine has been approved for emergency use by the World Health Organisation, after the European Medicines Agency issued its approval last week.
It’s the first single-dose vaccine to be authorised by the WHO, and while it must be stored at -20°C for most of its two-year shelf life, it can be kept at 2-8°C for up to three months.
The Pfizer/BioNTech vaccine can be stored at -20°C for up to two weeks, as long as it is kept at the required -90°C to -60°C the rest of its six-month shelf life, says the Therapeutic Goods Administration.
The company recently submitted data to the US Food and Drug Administration showing the vaccine maintains its efficacy even when stored at the higher temperatures for a short period of time.
The updated conditions mean the vaccine can be stored in standard pharmaceutical freezers for up to 14 days, even during transportation.
In Brisbane, a healthcare worker at the Princess Alexandra Hospital in Brisbane has tested positive for SARS-CoV-2 on March 11 after working with COVID-19 patients on March 10.
The hospital is in lockdown, and health alerts have been issued for the Morning After Café in West End, Corporate Box Gym in Greenslopes and Stones Corner Hotel in Stones Corner.
A security guard working hotel quarantine in Sydney has also tested positive for SARS-CoV-2 in a routine screen, prompting alerts for locations in Hurstville and the Sydney CBD.
The guard worked at the Sofitel Wentworth and the Mantra Sydney Central, and dined at Pancakes on the Rocks in Beverley Hills on Saturday March 13, which is considered a close-contact venue.
Here are the latest COVID-19 infection numbers from around Australia to 9pm Sunday:
National – 29,117 with 909 deaths
ACT – 123 (0)
NSW – 5237 (3)
NT – 105 (0)
QLD – 1380 (1)
SA – 631 (0)
TAS – 234 (0)
VIC – 20,483 (0)
WA – 924 (1)