Older adults are at risk of being excluded from around half of all COVID-19 clinical trials, study finds.
Welcome to The Medical Republic‘s COVID Catch-Up.
It’s the day’s COVID-19 news in one convenient post. Email bianca@biancanogrady.com with any tips, comments or feedback.
1 October
- Older adults excluded from many COVID-19 clinical and vaccine trials.
- Rapid antigen testing not recommended for clinical use in Australia, says RCPA.
- Cardiac arrest in COVID-19 associated with worse outcomes than cardiac arrest without COVID-19, study finds.
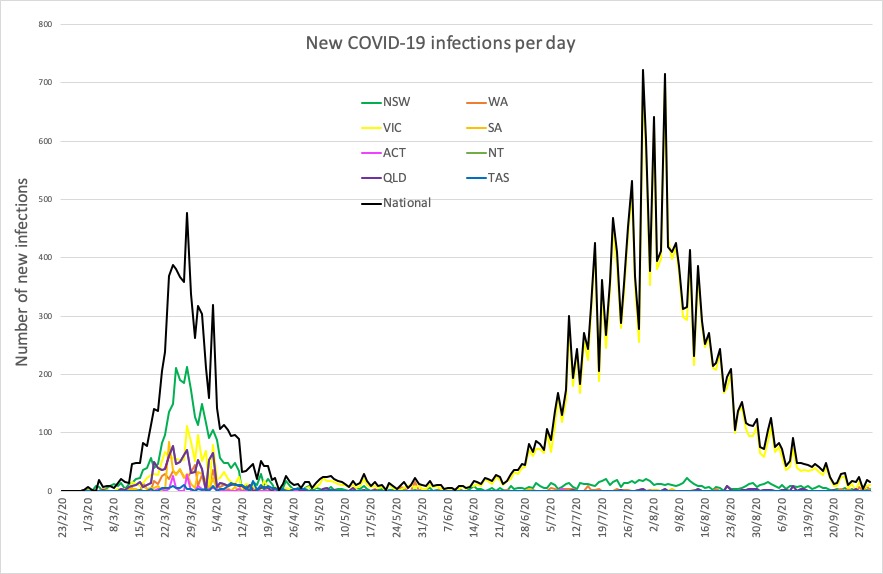
- Latest confirmed COVID-19 infection numbers from around Australia.
- Older adults are at risk of being excluded from around half of all COVID-19 clinical trials, including vaccine trials, research suggests.
Writing in JAMA Internal Medicine, researchers reported their analysis of 847 clinical trials, which revealed that nearly one-quarter had a specific age cut-off for participants. However many had more indirect age-related exclusions that would have disproportionately affected older adults, including specific comorbidities, technology requirements and compliance concerns. When these additional exclusions were taken into account, 53% of trials were considered to be high-risk for excluding older adults.
Drilling further into subsets of trials showed that half of the 232 phase 3 clinical trials and all of the 18 vaccine trials – including 61% that had age cut-offs for participants – were at high risk of excluding older adults.
“If the older age group is excluded from vaccine trials, efforts to ensure effectiveness, titrate dosage or frequency, and assess adverse effects in the group most vulnerable to COVID-19 will not be possible,” the authors wrote. - RT-PCR is still the preferred testing method for SARS-CoV-2 infection, says the Royal College of Pathologists of Australasia, which has advised against the use of rapid antigen tests in clinical practice.
In a statement, the College said that rapid antigen tests – which detect SARS-CoV-2 proteins, rather than viral RNA, in nasopharyngeal swabs – are not considered suitable for widespread use, particularly in regions with a low prevalence of COVID-19, because of their lower sensitivity and the risk of false negative results. The test relies on high viral load, so the risk of false negative may be higher in asymptomatic individuals or those who present more than 5-7 days after infection. - Cardiac arrest in patients with COVID-19 is associated with significantly higher mortality rates than in the absence of COVID-19, a study has found.
A study published in JAMA Internal Medicine examined outcomes in 54 patients admitted to hospital with COVID-19 who experienced a cardiac event and underwent CPR.
Historical data from before the pandemic showed one-quarter of patients who had an in-hospital cardiac arrest survived to discharge – despite the initial rhythm being non-shockable in 81% of cases.
However none of the COVID-19-positive patients who underwent CPR for cardiac arrest survived to discharge. All but two had non-shockable initial rhythm, 44 of them had pulseless electrical activity and 8 had asystole. While 29 patients experience return of spontaneous circulation, 15 of these then had their code status changed to ‘do not resuscitate’, and 14 recoded but died despite additional CPR.
Most of the patients were African American, nearly 80% were on mechanical ventilation, one-third were receiving kidney replacement therapy, and 46% were on vasopressor support.
The authors commented that the outcome among this group of patients was likely to be poor, given so many had unshockable rhythm.
“These outcomes warrant further investigation into the risks and benefits of performing prolonged CPR in this subset of patients, especially because the resuscitation process generates aerosols that may place health care personnel at a higher risk of contracting the virus,” they wrote. - Here are the latest confirmed COVID-19 infection numbers from around Australia, to 9pm Wednesday:
National – 27,078 with 886 deaths
ACT – 113 (0)
NSW – 4224 (4)
NT – 33 (0)
QLD – 1157 (0)
SA – 468 (0)
TAS – 230 (0)
VIC – 20,169 (13)
WA – 684 (0)