A study has cast doubt on the efficacy of remdesivir as a treatment for COVID-19, finding its effects as being “of uncertain clinical importance”.
Welcome to The Medical Republic‘s COVID Catch-Up.
It’s the day’s COVID-19 news into one convenient post. Email bianca@biancanogrady.com with any tips, comments or feedback.
24 August
- Uncertain clinical findings from new remdesivir study.
- US FDA issues emergency use authorisation for convalescent plasma despite mixed study results.
- Guidelines on protecting and respecting human rights of people with disabilities during COVID-19.
- Cruise ship study finds around half of COVID-19 cases are asymptomatic.
- Second quarantine hotel security guard tests positive to COVID-19 in Sydney.
- Latest confirmed COVID-19 infection figures for Australia.
- A study has cast doubt on the efficacy of remdesivir as a treatment for COVID-19, finding its effects as being “of uncertain clinical importance”.
The open-label study, published in JAMA, randomised 584 patients hospitalised with COVID-19 to either a 10-day or 5-day course of the antiviral, or to standard care.
The primary endpoint was the distribution of clinical status by day 11, with the idea being that if remdesivir did improve outcomes, then patients treated with it should have higher clinical status scores.
The study found that those treated for 5 and 10 days had significantly higher odds of having a better clinical status distribution than those treated with standard of care, but the authors were unsure how this translated clinically. They saw no significant differences between the three treatment groups in time to clinical improvement, time to recovery, time to discontinuation of oxygen support, duration of oxygen therapy or duration of hospitalisation. All-cause mortality at day 28 was 1% in the 5-day group, 2% in the 10-day group, and 2% in the standard care group.
They also saw a significantly higher rate of adverse events in the 10-day remdesivir group compared to the standard care group.
An accompanying editorial commented that there have now been three randomised controlled trials of remdesivir in hospitalised patients, and each has delivered different results, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped.”
The editorial’s authors also noted the considerable cost of producing and distributing remdesivir, and that its benefits compared to the widely available and much cheaper corticosteroids were unknown. - The US Food and Drug Administration has issued an emergency use authorisation for convalescent plasma, but numerous experts argue there’s not yet enough evidence for its efficacy.
Transfusions of plasma from individuals who have recovered from COVID-19 caught attention early on in the pandemic, with data from small non-randomised, non-controlled studies suggesting possible improvements in clinical symptoms. However a more recent larger randomised study found no benefits from the transfusions.
The Australian ASCOT study recently added a convalescent plasma air to investigate whether there is any benefit, but in the meantime Trump has jumped the cannula and declared this the Next Big Thing in fighting COVID-19.
The Emergency Use Authorisation allows convalescent plasma to be used in the US to treat patients hospitalised with suspected or confirmed COVID-19. - The Australian Human Rights Commission has issued guidelines for healthcare workers to ensure that the human rights of people with disabilities are not set aside in the healthcare response to the COVID-19 pandemic.
The guidelines cover scenarios such as the use of mobility aids which might otherwise not be permitted because of infection control, access to communication aids such as Auslan interpreters, and access to visitors and family.
“The purpose of the Guidelines is to provide practical guidance on how to apply a human rights-based approach to decision-making within the health system in the context of the current pandemic, that takes the rights of people with disability properly into account,” the guidelines authors write. - Another cruise-ship study has found the rate of asymptomatic infections ranged from 45% to 58%.
The study, published in Emerging Infectious Diseases, looked at an outbreak aboard a cruise ship docked in Yokohama, Japan, which affected around one in five of the 2666 passengers and nearly 14% of the 1045 crew members. Among passengers, nearly 58% were asymptomatic and among the crew, nearly 45% were asymptomatic. There were seven deaths among passengers, but none among the crew.
Because of the difficulty with isolating passengers and crew, the basic reproduction rate of the outbreak was four times higher than that seen in Wuhan, China. - A second security guard at the Sydney Harbour Marriott Hotel, which is quarantining returned travellers, has tested positive for COVID-19. The first guard infection was reported on 17 August, and genetic sequencing of the virus has connected it to one of the hotel guests. The second guard reportedly had no contact with that guest but genetic sequencing is underway to determine the likely source of their infection.
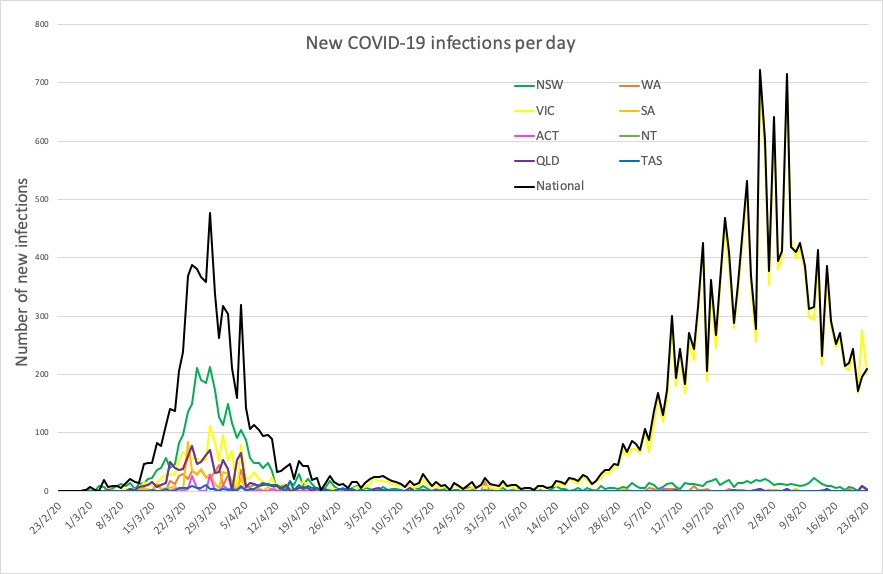
- Here are the confirmed COVID-19 infection numbers around Australia, to 9pm Sunday:
National – 24,812, with 502 deaths
ACT – 113 (0)
NSW – 3985 (4)
NT – 33 (0)
QLD – 1105 (2)
SA – 463 (1)
TAS – 230 (0)
VIC – 18,231 (208)
WA – 652 (1)