And while Pfizer's protection against infection wanes over time, protection against hospitalisation remains high.
Welcome to The Medical Republic‘s Covid Catch-Up.
It’s the latest covid-19 news in one convenient post. Email bianca@biancanogrady.com with tips, comments or suggestions.
5 October
- Interim analysis suggests molnupiravir halves risk of hospitalisation and death in at-risk adults.
- Pfizer protection against infection wanes over time, but protection against hospitalisation remains high.
- All Australians aged over 18 now eligible for any registered vaccines.
- TGA officially ‘recognises’ Coronavac and Covishield vaccines.
- Covid vaccination should be mandatory among all healthcare workers, says AHPPC.
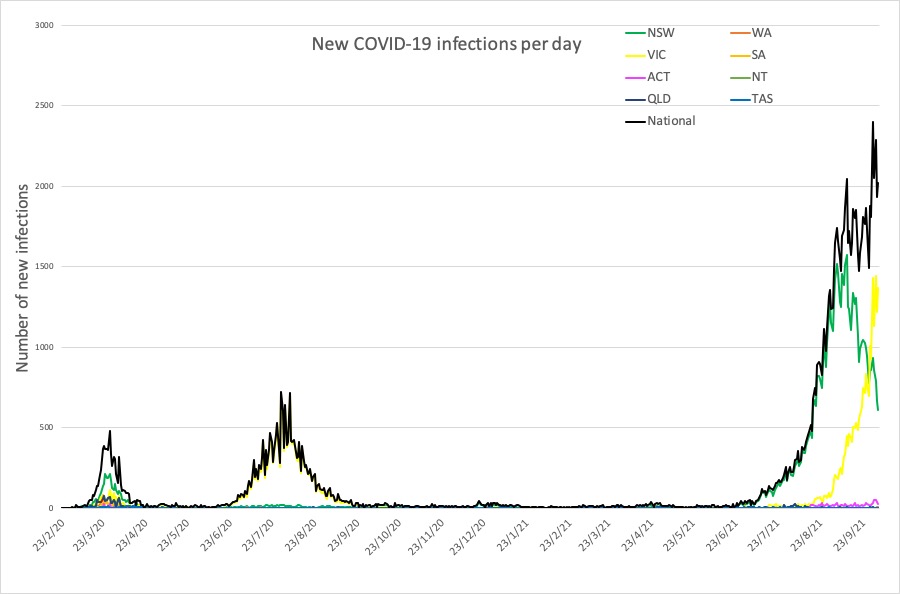
- Latest covid infection numbers from around Australia.
A new oral antiviral is generating considerable buzz after press-released data suggested it halved the risk of hospitalisation and death from covid among high-risk adults with mild-to-moderate disease.
The drug molnupiravir is being studied in an international phase 3 randomised, placebo-controlled, double-blind trial involving adults with at least one risk factor for severe disease, including obesity, older age, diabetes and heart disease.
Interim analysis of data (which is yet to be published) from 775 patients found that 7.3% of those in the treatment arm were hospitalised – but none died – in the 30 days since randomisation, compared to 14.1% of those given placebo who were hospitalised or died. The drug appeared to be equally effective across all variants, including the Delta variant, and the rate of adverse events was similar across the two groups.
The drug is being considered for emergency use authorisation in the US, and in Australia the Therapeutic Goods Administration has issued a provisional use determination, meaning that the manufacturer can apply for registration.
However the Australian government has leaped on the possibility of an antiviral drug against covid, and reportedly purchased 300,000 doses of the drug pending its approval, according to the ABC.
While the effectiveness of two doses of the Pfizer covid vaccine in preventing infection does wane over time, it still offers significant protection against hospitalisation at six months after the second dose, research suggests.
A retrospective cohort study, published in the Lancet, detailed PCR-confirmed infection and hospitalisation rates among more than 3.4 million people, just over one million of whom had received two doses of the Pfizer vaccine and around 76,000 had received one dose.
Over the entire study period, two doses of the Pfizer vaccine was found to be 73% effective at preventing SARS-CoV-2 infection and 90% effective at preventing covid-related hospital admission. The highest effectiveness in preventing infection was seen in those aged 12-15 years, where the vaccine was 91% effective, and the lowest was among those aged 65 and older, where the vaccine was 61% effective at preventing infection.
Over six months from the second dose of vaccine, protection against infection declined from 88% during the first month to 47% after five months. However the level of protection against hospitalisation remained stable, from 87% at one month to 88% at five months after vaccination.
The study also looked at the vaccine’s effectiveness against Delta variant. One week or more after the second dose, the Pfizer vaccine was 93% effective in preventing hospitalisation with covid and 75% effective at preventing infection.
At five months, protection against infection with Delta had declined to 53%, but the study did not report on whether protection against hospitalisation with Delta infection waned over time, as has been suggested by population-based data from Israel.
“Our results show high effectiveness of BNT162b2 against hospital admissions up until 6 months after being fully vaccinated in a large, diverse cohort under real-world vaccination conditions, even in the face of widespread dissemination of the delta variant,” the authors wrote.
Any Australian over the age of 18 years can now have whatever approved covid vaccine they want (and can get hold of), says the federal health department.
The Pfizer, Moderna and AstraZeneca vaccines are now available to anyone, even those aged over 60 years who were previously restricted to the AstraZeneca vaccine. Those aged 12-17 are only eligible for the Pfizer or Moderna vaccines.
The Therapeutic Goods Administration says both China’s Coronavac (Sinovac) and India’s Covishield are officially ‘recognised’ as covid vaccines, which is a step towards international travellers vaccinated with either of those to be admitted into the country.
In a statement, the TGA concluded there was enough evidence for the efficacy and safety of these vaccines that they be recognised. However four other vaccines – BBIBP-CoV and Convidecia from China, Covaxin from India and Sputnik V from Russia – are yet to be granted similar recognition.
Covid vaccination should be a mandatory condition of employment for all healthcare workers, says the Australian Health Protection Principal Committee.
AHPPC is recommending all workers in healthcare settings be vaccinated with two doses of a TGA- approved covid vaccine by 15 December 2021. The organisation has also called for a national definition of what constitutes a healthcare setting to ensure consistency across the country.
While acknowledging that vaccination requirements could reduce the availability of staff in some settings, AHPPC said a highly-vaccinated workforce would mean much lower transmission of covid in healthcare settings and reduce the need to quarantine and furlough staff.
Here are the latest covid infection numbers from around Australia to 9pm Monday:
National – 113,411 with 1344 deaths
ACT – 1129 (28)
NSW – 65,279 (611)
NT – 212 (0)
QLD – 2046 (3)
SA – 906 (1)
TAS – 236 (0)
VIC – 42,494 (1366)
WA – 1109 (10)