An insulin-delivery device using excess blood glucose as fuel is a beautiful solution in search of funding.
“They got funding for this?” is a question that flits through the mind fairly often when reading research articles.
“Somebody please fund this!” is a thought that occurs far less frequently.
But such is your Back Page correspondent’s reaction on reading about this very elegant invention that harnesses the central problem of diabetes to solve itself.
Led by ETH Zurich biotechnology and bioengineering professor Martin Fussenegger, a team has developed an implantable fuel cell that converts excess blood glucose into power that drives engineered beta cells to produce insulin.
To date, bioelectronic implants require too much energy to run on batteries, and need to be wirelessly powered by external generators. This invention turns the person themself into a battery during their bouts of hyperglycaemia.
Tapping into blood glucose as an energy source to power such a device is a clear stroke of genius, especially if lowering blood glucose is the aim of the device in the first place.
This team didn’t invent the idea of electro-metabolic conversion, but previous attempts have had problems including “low power output, limited electron transfer, poor shelf/half-life of the enzyme and biofouling of implanted electrodes”; consuming oxygen, “which promotes hypoxia and electrode corrosion, renders them useless for clinical application”; or only working at glucose concentrations far higher than found in even diabetic patients.
The team have conquered these problems, they say, with their “mediator-free metabolic fuel cell that constantly monitors blood-glucose levels, exclusively produces electricity during hyperglycaemia and generates sufficient electricity to power and control optogenetic-as well as electrogenetic-based bioelectronic implants. By programming these bioelectronic implants for rapid release of insulin from engineered human cells … we have established closed-loop metabolic control systems that can autonomously restore glucose homeostasis”.
The same group had already developed artificial cells a few years ago that mimic the function of insulin-producing pancreas beta cells.
The fuel cell, which resembles a small tea bag
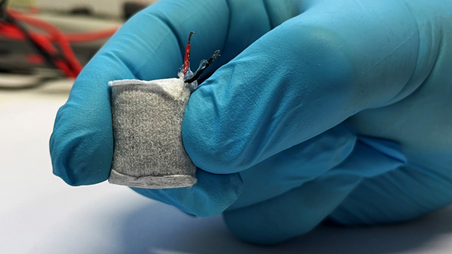
is then hooked up to a capsule containing the engineered cells, which are stimulated by electric current or blue LED light to secrete insulin.
The catch: they so far have only a prototype and have only tested the tech in vivo in mice, and require significant funding to take things to the human level.
To The Back Page’s untutored eye, this looks like a project worth flinging some cash at.
Sending story tips to penny@medicalrepublic.com.au keeps your blood glucose within the acceptable range.


