Integrated-care models can mean quicker diagnosis, particularly for patients with complex hearing issues
In 2016, the World Health Organisation estimated the economic cost of hearing loss to be $750 billion globally,1 equalling the combined annual health expenditure of Brazil and China.
Worldwide, the disability adjusted life years for sense organ diseases is increasing, now being the seventh-most burdensome disease in the world.2 In Australia, the disease burden in the indigenous population is especially high, with less than 10% of Aboriginal children below the age of three in the Northern Territory having normal ears.
One can only anticipate, therefore, what the global economic burden is for hearing and balance disorders combined. Patients with vestibular disorders typically rate their quality of life as impacted more by their imbalance than their hearing loss.
Despite technological advancements in the area of diagnostic testing, rehabilitative strategies and surgical techniques, the overall cost of treatment is minor compared with the overwhelming cost of the disease burden when awareness and access to specialist healthcare remains poor.
As healthcare professionals, we therefore need to integrate our care models such that care for hearing and balance disorders is provided within a streamlined pathway.
General practitioners are at the forefront of healthcare, and awareness of current approaches to the management of hearing and balance disorders is encouraged, to facilitate the provision of information to patients and to treat or direct them to appropriate services.
This article demonstrates the utility of some modern approaches to the investigation and management of such patients.
Case history
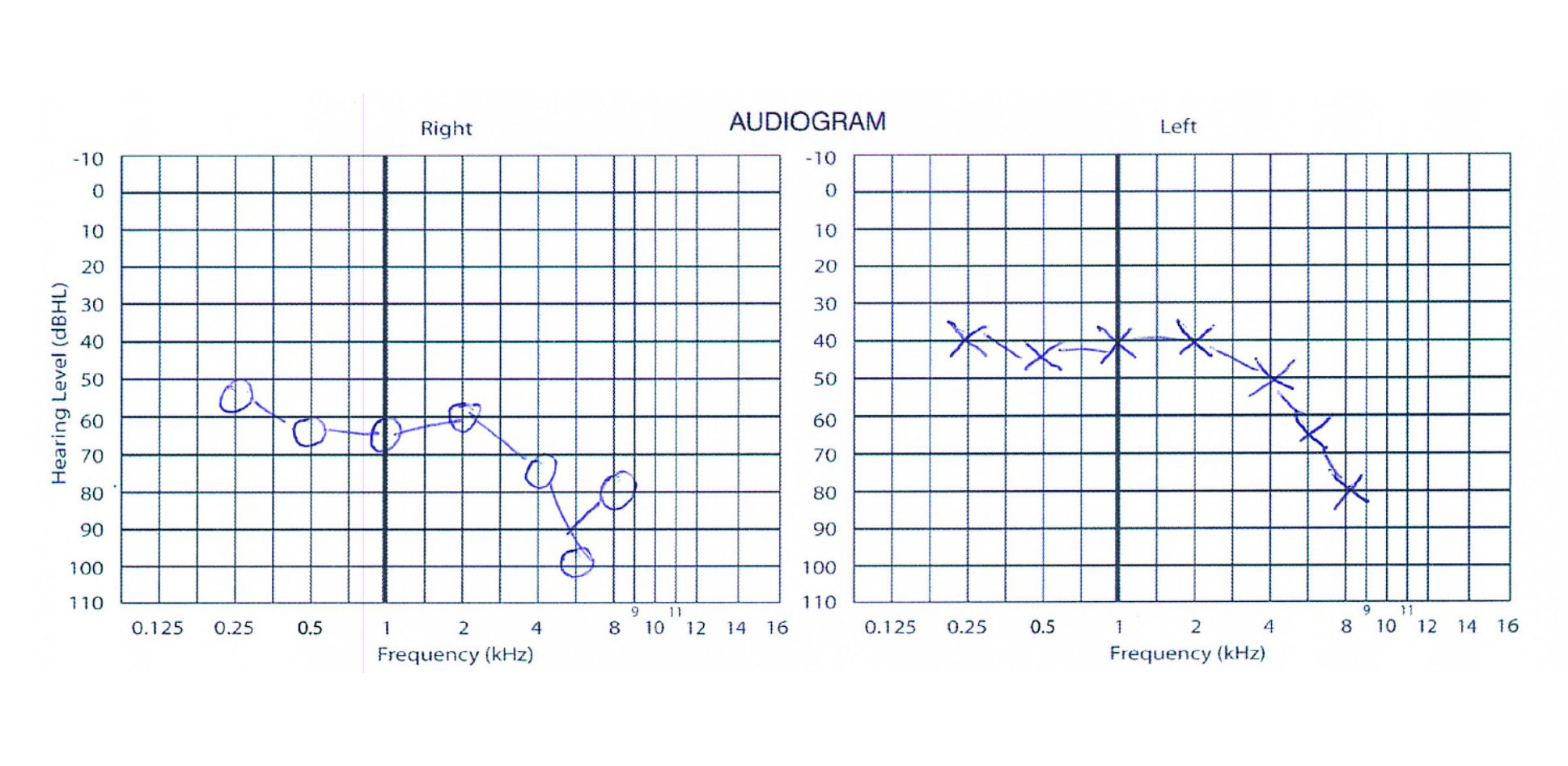
Bob, a 76-year-old male presented with worsening vertigo on the background of a five-year history of right-sided deafness and tinnitus. Clinical examination was unremarkable. Diagnostic testing performed by the audiologist included an audiogram (See figure 1, page 36), tympanogram, word speech tests, electrocochleography and vestibular function testing (video head impulse testing, vestibular evoked myogenic potentials and caloric testing, which tests the function of the lateral semicircular canal).
Testing raised the possibility of bilateral Meniere’s disease, even though he had symptoms of aural fullness and tinnitus only in the right ear. This diagnosis is typically reached with an abnormal electrocochleography result indicating hydrops in the cochlea or the combination of an abnormal caloric and normal video head impulse test which may be seen when hydrops is present in the lateral semicircular canal. Imaging excluded a vestibular Schwannoma and middle ear or mastoid disease. He was optimised medically and his vertigo improved.
Following this, the priority became optimising Bob’s hearing deficits. Although he had bilateral hearing loss, his right ear was worse (See figure 1) and he complained of reduced clarity and increased distortion from his right hearing aid.
Bob underwent speech testing with and without hearing aids which involved assessing what percentage of speech he understood in words and sentences with and without a hearing aid, in a quiet environment and also with background noise. This is part of routine cochlear implant candidacy assessment, the principle of which is to ascertain whether the cochlea is not working when hearing aids are optimally fitted and the patient is still not attaining usable speech understanding in his or her natural environment.
Speech comprehension in Meniere’s patients can often be worse than expected, despite an acceptable audiogram.
The audiogram (Figure 1) would suggest that Bob should get good benefit with hearing aids. However, as can often be seen in patients with Meniere’s disease, his speech scores showed that even with the most optimised hearing aid in the right ear he was only understanding 28% of words on CNC speech test (consonant-vowel nucleus consonant) and 56% of sentences on BKB speech test (Bamford-Kowal-Bench).
Furthermore, a period of observation had confirmed that his hearing was not fluctuating. It was clear, therefore, that Bob’s cochlear function was impaired and that he was a potential candidate for cochlear implantation in his right ear. One would expect that with cochlear implantation, he would be able to attain greater than 70% speech understanding in the same tests.
In addition to his vertigo and hearing deficit, Bob also experienced three falls within a period of two weeks, which resulted scalp lacerations, signifying he had developed a dangerous, albeit rare, variant of Meniere’s disease called drop attacks.
Because of the complexity of his disease, his management was centralised to a multidisciplinary hearing and balance centre where he could access all care givers for his Meniere’s disease, including cochlear implant surgeon, audiologist, vestibular physiotherapist and also vestibular researchers under one roof.
Despite his age, Bob was in otherwise good health and the primary driver in the household. His drop attacks meant that it was too dangerous to drive and this caused significant impairment of his quality of life.
It was determined that Bob needed a labyrinthectomy to permanently relieve him of symptoms of drop attacks but since this would cause permanent loss of hearing, he would undergo simultaneous cochlear implants as well.
Discussion points
There were three aspects to this patient’s care which were equally important and required team discussion and co-ordination.
1. Which ear was the problematic side? Due to the concern about Meniere’s disease bilaterally, it was crucial to pick the correct ear on which to perform the labyrinthectomy. Failure to do so would risk bilateral vestibular failure and oscillopsia (a sense of visual field moving up and down with movement). Equally, a labyrinthectomy, though making Bob safe and ridding him of his drop attacks, would permanently deprive him of vestibular function in that ear. Given he had developed Meniere’s in the other ear, this meant he could still lose vestibular function in the other side. A detailed team discussion was necessary to ensure that Bob’s right ear, which was the most symptomatic ear, was also the correct ear for surgery. Intratympanic gentamicin (a less destructive option) was discussed with him, which he declined.
2. Physiotherapy. Bob was expected to experience vertigo immediately following labyrinthectomy, needing specialist vestibular physiotherapy for rehabilitation both as an inpatient and an outpatient. Team discussion revealed that he would be a good candidate for “prehab” i.e. his physiotherapy commenced preoperatively. Preoperative training and education allowed him to commence his exercises autonomously postoperatively, rather than having to learn them when he was dizzy and feeling unwell.
3. Co-ordination of his cochlear implant rehabilitation with the audiologist, vestibular rehabilitation and physiotherapist. Following surgery, Bob underwent ongoing physiotherapy, and co-location with the surgeon allowed him to access the surgeon immediately, if necessary, when visiting the audiologist for his implant rehabilitation or the physiotherapist for vestibular rehabilitation. With a chronic condition such as Meniere’s disease, this allowed each of the caregivers to provide optimal care, as multi-disciplinary discussions and specialised decision making could be expedited.
This case highlights the value of a multidisciplinary care model in co-ordinating patient care. Such models are relatively new, but serve to benefit both clinician and patient by improving communication between caregivers, reducing patient visits to various caregivers, and thereby reducing frustration and cost and saving time.
Speech comprehension in Meniere’s patients can often be worse than expected despite an acceptable audiogram. This is because an audiogram doesn’t test the complexity of function in a patient’s normal environment.
If a patient complains of dissatisfaction with their hearing aids, clinicians should have a low threshold to refer for a cochlear implant candidacy assessment.
With improved understanding of hearing conditions and improved cochlear implant technology, two changes to candidacy affect Meniere’s patients:
1. Single sided hearing loss is now considered a candidacy criteria for a cochlear implant. This means a patient no longer needs to be deaf in both ears to be considered a candidate. Each ear is assessed individually.3-5
2. A labyrinthectomy is not considered a contraindication for cochlear implant. In fact, patients undergoing labyrinthectomy can be implanted simultaneously, bringing permanent relief to vertigo while improving hearing at the same time.6-8
Meniere’s patients usually derive excellent speech discrimination with cochlear implants because their hearing loss is primarily due to cochlear hair cell loss, and implantation bypasses these hair cells and stimulates the cochlear nerve directly.9,10
Meniere’s patients usually derive excellent speech discrimination with cochlear implants because their hearing loss is primarily due to cochlear hair cell loss.
Drop attacks, or Tumarkin’s crises, are caused by a disorder in the otolithic organs which are responsible for horizontal or vertical movement. They present with an incidence of 5% to 10% in Meniere’s patients. Especially in the elderly, a severe drop attack may present as a sudden fall without loss of consciousness and cause serious damage. Definitive treatment, such as a labyrinthectomy, is warranted especially if the attacks are severe.11
Bob’s outcome
With the multidisciplinary clinic situated on site at the hospital, Bob’s cochlear implant rehabilitation was expedited. In the past, cochlear implant switch was routinely performed three weeks post operatively. The patient routinely would see the surgeon at one week post operatively for a wound check, then travel to another clinic for the “switch on” with his audiologist at three weeks, and again at yet another clinic for ongoing review with the physiotherapist.
However, in this case, Bob’s cochlear implant was “switched on” prior to his discharge from the hospital and he was mobilising independently with a walking stick within 48 hours due to the preoperative training with the physiotherapist.
He saw his audiologist, surgeon and physiotherapist in the same visit post operatively, once again expediting his care, but also saving him the frustration of numerous visits.
Summary
Working in a multidisciplinary team is crucial for the optimal outcome of such patients as Bob, especially given that as technological advancements are made, and more specialised care is available, it is important to address access and availability to this care. Equally, such a structure allows clinicians to improve their outcomes for complex patients through better communication, focused testing, reduced duplication of tests and further research into these multifaceted problems through team work.
Given the aging population of Australia, multifactorial problems are common and if a collective approach can be instituted in a multidisciplinary team, patients may enjoy a quicker diagnosis and more effective management leading to recovery and a better quality of life.
Dr Payal Mukherjee is senior clinical lecturer at University of Sydney; adult and paediatric ENT surgeon; otologist; and cochlear implant and skull base surgeon
References:
1. WHO report 2016: Action for hearing loss: make a sound investment 2017
2. GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1603-58.
3. Mukherjee P, Eykamp K, Brown D, Curthoys I, Flanagan S, Biggs N, McNeill C, Gibson W. Cochlear Implantation in Meniere’s Disease with and without labyrinthectomy. Otology and Neurotology. 2017; 38(2):192-198
4. Kamal S, Robinson S, Diaz R. Cochlear Implantation in single sided deafness for enhancement of sound localisation and speech perception. Current Opinion. 2012; 20: 393-97.
5. Tavora-Vieira D, Marino R, Krishnaswamy J, Kuthbutheen J, Rajan G. Cochlear Implantation for unilateral hearing loss with and without tinnitus: a case series. Laryngoscope 2013; 123: 1251-55.
6. Osborn HA, Yeung R, Lin VY. Delayed cochlear implantation after surgical labyrinthectomy. Journal of Layrngology and Otology. 2012; 126: 63-65.
7. Chen D, Linthicum JR and Rizer F. Cochlear histopathology in the labyrinthectomised ear: Implicatations for Cochlear Implantation. Laryngoscope. 1988; 98: 1170-72
8. Wareing M, O’Connor AF. The role of labyrinthectomy and cochlear implantation. Ear Nose and Throat Journal. 1997; 76 (9): 664-9.
9. Lustig LR, Yeagle J, Niparko JK, et al. Cochlear implantation in patients with bilateral Ménière’s Syndrome. Otol Neurotol. 2003; 24:397-403
10. Doobe G, Ersnt A, Ramalingam R, Mittmann P, Todt I. Simultaneous Labyrinthectomy and Cochlear Implantation for Patients with Single-Sided Ménière’s Disease and Profound Sensorineural Hearing Loss. Biomed Res Int. 2015. Online publication. Article ID 457318, 4 pages.
11. Vibert D, Caversaccio M, Rudolf H. Meniere’s Disease in the Elderly. Meniere’s disease in the Elderly. Otol Clin N Am. 2010; 43: 1041-46.