New Australian research suggests CGRP monoclonal antibodies have real-world efficacy for up to 12-months in complex patients.
Recent research supports the effectiveness of monoclonal antibody migraine treatment but shows it may take longer than three months to work.
CGRP monoclonal antibodies have revolutionised the way we treat migraine, but questions about their generalisability remain due a lack of long-term data and research in broader populations.
Now a new Australian multicentre cohort study, published in BMJ Neurology Open, shows 80% of chronic migraine patients taking galcanezumab and fremanezumab had a ≥ 50% reduction in headache days after 12 months. This corresponded to having a median 18 more headache-free days per month.
“These findings provide further evidence of the safety and efficacy of CGRP monoclonal antibodies over a longer time-period, and critically, in cohorts of patients relevant to Australian practice who were excluded from the original clinical trials – particularly patients with continuous pain [or those who had] trialled a higher number of previous preventative therapies,” said lead author Dr Jason Ray, a neurologist from Alfred Health in Melbourne.
Related
Researchers prescribed galcanezumab or fremanezumab to 105 chronic migraine patients, who completed headache diaries and were followed up on a quarterly basis for 12 months.
The primary outcome was at least 50% or greater response – the proportion of patients who had at least a 50% reduction in the number of headache days per month compared to their pre-treatment baseline.
Fifty-two percent of patients achieved a 50% or more reduction in headache frequency after three months, corresponding with a median decrease of 10 headache days per month. The 50% or more response rate rose to 80% for patients still receiving treatment at 12-months.
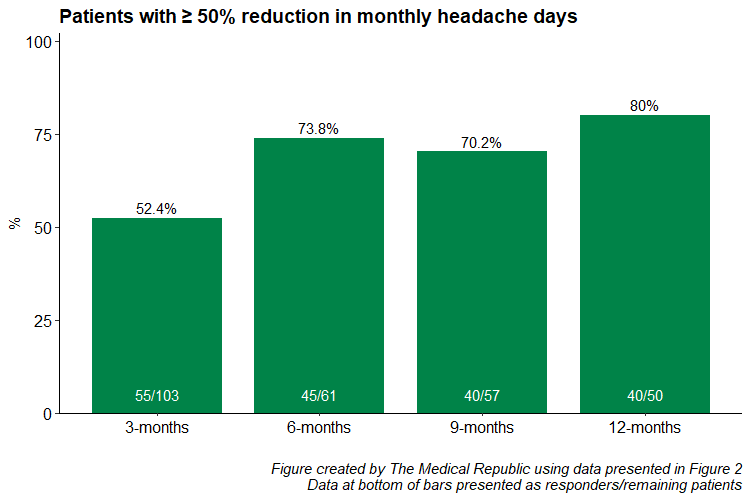
Twenty-one percent of patients who showed at least a 50% reduction in monthly headache days at 12 months had not shown this reduction at three months.
“There is increasing evidence there is a significant proportion of patients who we could help with this class of medication – and with other highly effective treatments such as botulinum toxin A – who are ‘late responders’,” Dr Ray told The Medical Republic.
“However [we] are unable to do so within the constraints of the PBS, which requires patients who do not achieve a 50% reduction [in monthly migraine days] within three months be discontinued from therapy.”
Dr Adele Stewart, chair of the RACGP pain management specific interest group, agreed the findings supported the concept of reviewing PBS requirements around ceasing treatment in non-responders.
“Poor agreement between the three-month and 12-month ≥50% response rates does highlight the need for further clinical studies to explore the three-month rule the PBS uses, as this may not be an adequate trial [period] to assess response,” said Dr Stewart.
Forty-two percent of patients had discontinued treatment at 12-months due to lack of efficacy, adverse effects or a change in circumstances. There was no difference between galcanezumab and fremanezumab with respect to reducing headache frequency.
Dr Ray acknowledged that GPs played a key role in managing chronic migraine and limiting the significant disability associated with the condition by addressing lifestyle factors, optimising acute treatments and starting preventative agents.
“Counselling for the risk of medication overuse headache and avoiding overuse of acute analgesia (triptans more than 10 days/month or simple analgesics more than 15 days/month) will help compounding disability,” Dr Ray said.
Both galcanezumab (Emgality) and fremanezumab (Ajovy) are TGA-approved and available via the PBS. GPs can continue scripts for galcanezumab and fremanezumab provided a neurologist provides the initial prescription.






