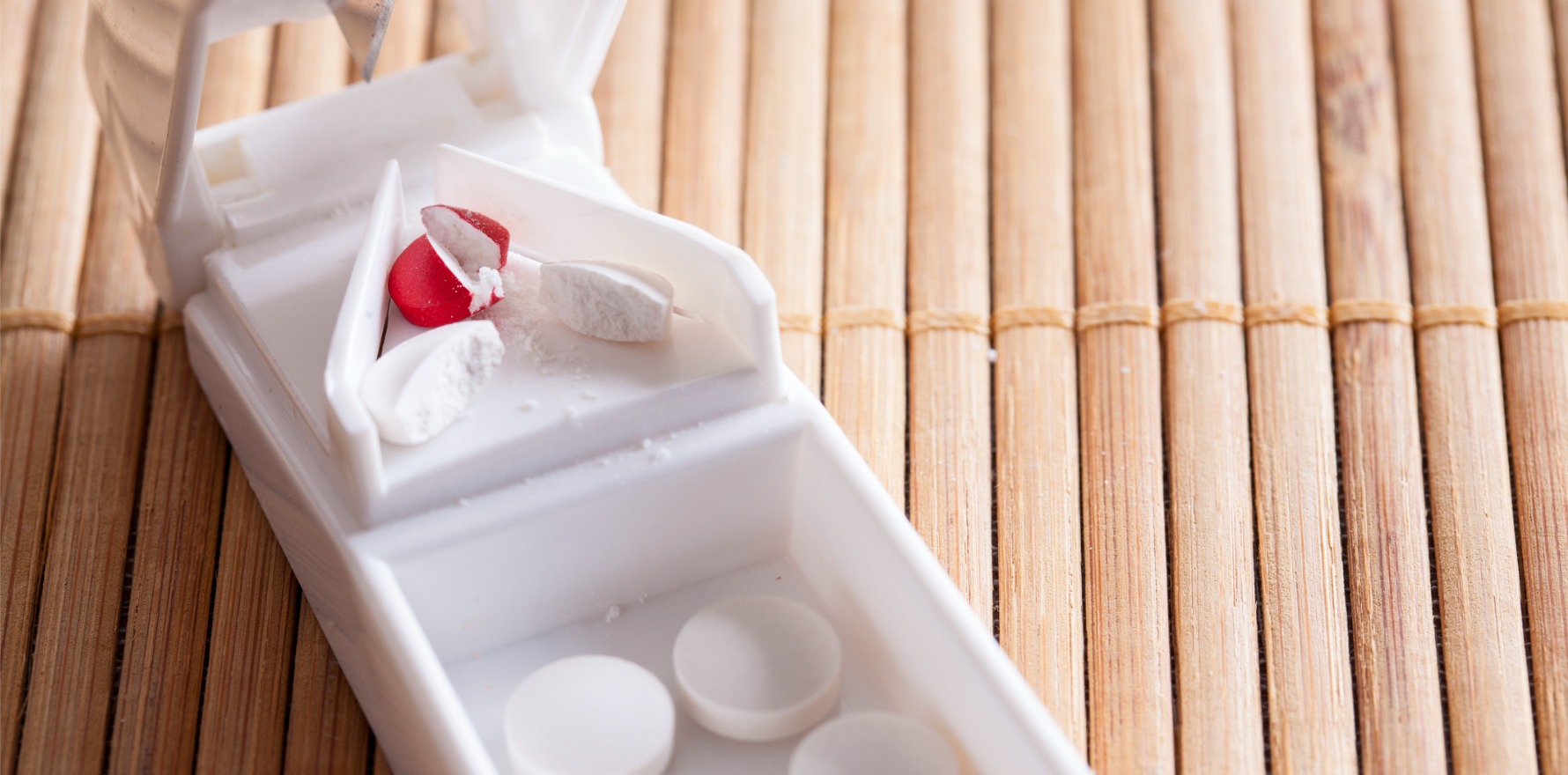
Researchers have called for paediatric formulations to reduce the risks that come with splitting adult tablets into child-size doses.
Australian researchers have called for paediatric formulations for ADHD medications after new figures show a large proportion of poisonings occurred from a medication that is only available in adult doses.
The researchers found that more than 17,000 exposures to ADHD medications were reported to the NSW Poisons Information Centre between 2014 and 2023.
Poisoning exposures to ADHD medications have grown 16.5% each year in that period, with more than 3000 exposures reported in 2023 compared with 795 in 2014.
More than half (56%) of all cases were either referred to hospital or already in hospital when the notification was made. Overall, 38.5% of exposures were intentional, mainly due to deliberate self-poisoning, the researchers said.
Among the six medications used to treat ADHD, clonidine and methylphenidate were the most commonly linked to poisoning, each causing 35% of reports.
“Poisoning exposures to ADHD medications present a growing public health issue,” the researchers said in the Australian & New Zealand Journal of Psychiatry.
Lead author and PhD candidate at the University of Sydney’s School of Pharmacy, Amy Thomson, said that the number of poisonings had increased proportionally with the number of ADHD medication prescriptions, but clonidine was an exception.
“The one that really stood out in our study was clonidine, where the number of poisonings and exposures has far outstripped the number of prescriptions written,” she told The Medical Republic.
Clonidine was also the most likely to result in hospital care, with 70% of exposures either referred to hospital or in hospital at the time.
Ms Thomson said one reason for the rise in clonidine poisonings was the lack of paediatric formulations for the medication, which is commonly used as a second or third-line therapy for ADHD.
“We’re using a tablet that was originally designed for adults, and it’s getting used in children for ADHD. It’s not first line, but it is still being used, and parents are needing to cut tablets in half or quarter, and so it’s a lot harder to get the correct dose for the child.”
Ms Thomson said the majority of clonidine poisonings in children were accidental.
“That’s often situations, for example, where a child has become curious or has tried to be independent and has taken additional tablets, or a whole tablet instead of a half unintentionally. There are also cases where there’s been miscommunication in the household and both parents have dosed the child.
“We know that it’s got a very narrow therapeutic window, and as little as a double dose can result in the child needing to go into hospital. If we are going to continue using this medication … there needs to be a proprietary paediatric formulation made available.”
In Queensland, GPs can diagnose ADHD and initiate medication, while in NSW, specially trained GPs can now write continuation scripts for ADHD medications. South Australia and Western Australia will introduce the same reforms next year.
Ms Thomson said GPs played a key role in communicating the risks and benefits of medications and the importance of medication safety.
Families need clear, open communication in the household about who has given what medicine and when, and to be aware of the risks of complicated dosing regiments in households, Ms Thomson said.
“Some of the cases that came up in our study where there were multiple children in the household who were prescribed ADHD medicines, and in the chaotic morning of the school run, the wrong medicine has been given to the wrong child, which had resulted in a poisoning.
“It’s a really good opportunity in the GP consult if it’s a new medicine, but really at any time, making sure that those things are mentioned, so that people … can work out what works best for their household.
Related
“Some people are really good at keeping a notepad with the medicine and writing it down, or other people will text each other and they’ve got that kind of record. They’ll know their families best and what mechanism will help them avoid double doses.”
Ms Thomson said psychostimulant overdoses could agitation, cardiac ischaemia and seizures, while clonidine and guanfacine overdoses could cause drowsiness, hypotension, bradycardia and coma, sometimes requiring intubation.
“Clonidine in particular has a fairly rapid onset of effects, but tends to linger,” Ms Thomson said. “Quite often children who become symptomatic and need to go to hospital, end up in hospital overnight or for several days.”
While the number of Australian diagnosed with ADHD has doubled between 2013 and 2020, the researchers said that could only partly explain the increase in poisonings.
Clonidine had the highest rate of exposure, with 6.5 exposures per 1000 prescriptions, the authors said. Boys under one, girls aged five to 14 and girls aged 15 to 19 years were disproportionately represented, they said.
The researchers said that while the raw count of exposures in neonates and infants was small, “the presence of any exposure in these highly vulnerable age groups warrants attention and requires further investigation into the nature and the circumstances of exposure”.
The authors said 72% of accidental poisonings occurred in children under 15.
“As exposures closely tracked with dispensing, it is likely this trend will continue unless efforts are made to reduce availability of these medications in the home,” the authors said.
“Interventions include restrictions to pack sizes and quantities prescribed, better safety packaging, and a proprietary paediatric formulation for clonidine, such as a crushable sublingual tablet of low strength.”
The research comes as a new review in The BMJ confirms that taking paracetamol during pregnancy does not increase the risk of autism or ADHD in children.
To address uncertainly around existing reviews, the authors did an umbrella review of systematic reviews to examine the quality of evidence and the association between paracetamol use during pregnancy and the risk of autism or ADHD in babies.
They found that confidence in the findings of systematic reviews was “low to critically low”.
Any apparent effect could be explained by genetic or environmental factors and unmeasured confounders, they said.
“The current evidence base is insufficient to definitively link in utero exposure to paracetamol with autism and ADHD in childhood,” the Australian, UK and Spanish authors said.
They said patients should be informed about the poor quality of existing reviews, and the likelihood that positive associations reported in studies were driven by familial confounding.
Australian & New Zealand Journal of Psychiatry, 10 November 2025