What should doctors make of research showing honest placebos do provide real symptom relief?
It’s a counter-intuitive, mind-bending and rather uncomfortable fact that placebo pills produce about 60 to 80% of the benefit of the best medications for diseases with subjective symptoms such as chronic pain and depression.
That is, a patient with chronic low back pain, mild-to-moderate depression or a migraine can swallow a pill that contains nothing but microcrystalline cellulose and experience clinically relevant symptom relief over a prolonged period of time without any of the side-effects of active medication.
Doctors have always known this. Before the emergence of informed consent as a core principle of medicine in the mid-20th century, well-meaning physicians regularly hoodwinked patients with phoney treatments.
Former US president Thomas Jefferson described the practice as “pious fraud” in a letter to his friend Dr Caspar Wistar in 1807: “One of the most successful physicians I have ever known, has assured me, that he used more bread pills, drops of coloured water, and powders of hickory ashes, than all other medicines put together,” he said.
Today, such trickery is considered reprehensible, and doctors recoil from placebos. In fact, clinical trials are specifically designed to weed out medications that can’t outdo sugar pills.
Due to seemingly insurmountable ethical barriers, very few resources have been devoted to discovering how to maximise the placebo effect, despite it being a cheap and mostly harmless treatment.
In 2010, all that changed. That was the year Ted Kaptchuk, a professor at Harvard Medical School in the US, ran the world’s first open-label placebo trial, which challenged the assumption that placebos needed deception in order to work.
Looking back, Professor Kaptchuk describes his career-defining leap into the unknown as “perilously hasty”.
It all started with a hunch. Scraps of commentary from a placebo-controlled trial showed that patients weren’t duped into believing they were receiving a real drug just because they were feeling a little better.
The study participants were very aware they only had a 50% chance of receiving active medication, and they worried that their small respite from chronic illness might be “all in their head”.
“I began to wonder if it would be possible to reframe this hope, despair and apprehension and honestly discuss and prescribe placebos instead,” Professor Kaptchuk said.
It was an ‘ah ha!’ moment for the academic, who had struggled for a number of years with the “ethical frustration” of trying to make placebo research square with the moral principles of modern medicine.
“Ultimately, desperation to find an ethical clinical direction for my work fed my decision to risk scarce resources to investigate what most of my colleagues considered ridiculous,” he said.
Professor Kaptchuk would go on to make history by demonstrating that honest placebos could relieve symptoms in patients with a range of different chronic conditions.
“Our patients tell us it’s nuts,” he said in a recent interview. “The doctors think it’s nuts. And we just do it. And we’ve been getting good results.”
The first open-label placebo study involved 80 adults, each with a gastroenterologist-confirmed irritable bowel syndrome (IBS) diagnosis.
All study participants were explicitly told that the placebo pills did not contain any medication.
A health practitioner explained that it was not necessary to believe in the placebo treatment for it to work; the human body could automatically respond to the placebo through behavioural conditioning, associative learning and positive expectations (much like Pavlov’s dogs who salivated when they heard a bell).
Patients frequently expressed incredulity, as did some of the clinicians involved in the trial.
“It was obvious that most of our patients thought that knowingly taking placebos was ludicrous,” said Professor Kaptchuk. “We provided room for them to express their scepticism.”
The aim of this pre-trial discussion was to remove stigma from placebo effects. “We talked about an attitude of ‘let what happens happen’,” he said.
At three weeks, the placebo pills had worked their magic: 60% of open-label patients obtained “adequate relief” for IBS on a standardised questionnaire compared with 35% for no treatment. The differences were statistically significant.
“Our results challenge the conventional wisdom that placebo effects require intentional ignorance,” the researchers wrote in PLOS One.
For the next study, Portuguese researchers signed up 83 adults with specialist-confirmed persistent low back pain for a “novel mind-body clinical study”.
Patients were given the same spiel about placebos as those in the IBS trial. They also viewed a short video in which one participant of the previous IBS trial claimed the placebo pills worked “like a miracle”.
Half of the patients received a bottle filled with Swedish orange gelatin capsules containing an inert, white powder commonly used in pharmaceuticals. The bottle was clearly labelled “placebo pills – take two pills twice a day”.
The rest of the patients received no treatment.
Three weeks later, the patients taking placebo pills reported a statistically significantly 28% reduction in pain, whereas the no-treatment patients experienced a 5% reduction in pain. Adverse events were almost non-existent.
Everyone was instructed to keep taking their usual dose of NSAIDs or other medications throughout the trial, so the placebo effect was just the icing on the cake, so to speak.
Over the following few years, the outlandish results kept rolling in.
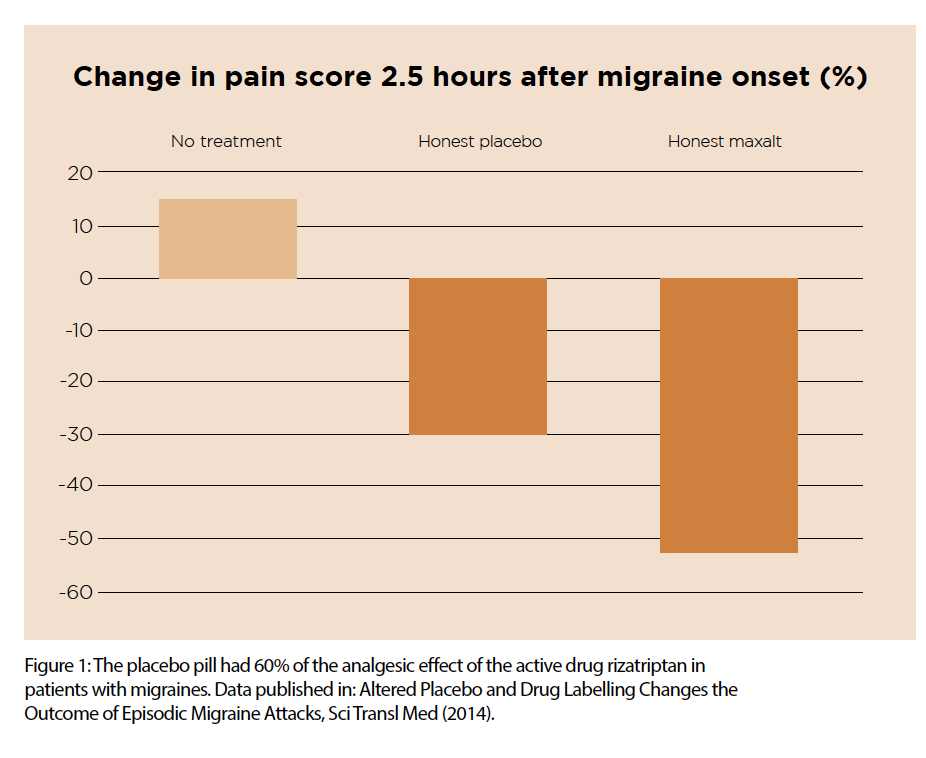
A 2014 study showed that placebo pills reduced migraine pain by 15% over two hours, compared with a 15% intensification of pain in patients on no treatment. In this study, the placebo pill had 60% of the analgesic effect of the active drug Maxalt (see Figure 1). And, astonishingly, there was no significant difference in pain relief between the placebo and rizatriptan, when each was disguised as the other.
A study on allergic rhinitis, published in 2018, showed that open-label placebos produced a significant reduction in symptoms such as sneezing, itching, running nose, eczema, breathlessness and cough, compared with no treatment.
Some patients in this trial were left in the dark about the mechanisms behind the placebo effect, whereas others were intentionally given more detail to raise their expectations. Curiously, the information context made no difference to the impact of the placebo; even poorly informed patients felt its effects.
The latest study, published this year, found that open-label placebos improved cancer-related fatigue scores by 29% over three weeks compared with 10% improvement in patients receiving treatment as usual.
If all this research is to be believed, a 15 to 30% health benefit is there for the taking; GPs just have to start openly prescribing sugar pills.
Before pharmacies start stocking up on dummy medicine, there are quite a few holes to poke in the empirical basis for such a radical proposal.
For starters, every one of these papers have Professor Kaptchuk’s fingerprints on them, which means the field of open-label placebo research, as it stands, has not yet been validated by an entirely independent research group.
The studies have small sample sizes and lack long-term follow up.
Patient know which treatment they are receiving, and symptoms are self-reported, which makes it impossible to know whether the placebo actually makes a patient feel better or whether it just makes the patient say they feel better.
Understandable biases, such as wishing to please the experimenter or wanting to answer questions politely, may be enough for a patient to slide their pain score up or down a few notches.
Most clinical trials would limit these biases by giving the control group a blinded placebo pill. Obviously, this can’t be done in a trial that is testing a placebo pill against no treatment.
However, the experimental design might have cancelled out some of the bias on its own, Dr Jeremy Howick (PhD), a placebo researcher and the director of the empathy program at the University of Oxford, said.
The no-treatment group were likely to get a placebo effect just through their engagement with concerned, caring clinicians, which would bring their scores closer to the open-label placebo group, he said.
On top of these obvious weaknesses, Cochrane’s 458-page treatise offered a crushing indictment of placebo research in 2010.
It found that blind placebos did not have important clinical effects in general.
The negative findings in the Cochrane review are bad news for honest-placebo researchers. (Blind placebos generally produce greater effects than open-label placebos, so if the blind placebo fails as a treatment, it is logical to predict that the honest placebo will also fail.)
The review found that deceptive placebos could influence patient-reported outcomes, especially pain and nausea, but that it was “difficult to distinguish patient-reported effects of placebo from biased reporting”.
Professor Kaptchuk speculated that lumping 202 trials covering 60 healthcare problems together, as the Cochrane review did, was a good way to bury any evidence of the positive effect that placebos might have for a narrow set of conditions.
“The Cochrane study does a heavy mixing of oranges and apples,” he said. “I think the reliable way to discuss is to go to the original papers and discuss what it means per indication.”
Fortunately for placebo advocates, there’s another meta-analysis that directly butts heads with the Cochrane review. The meta-analysis of open-label placebos, conducted by Dr Howick in 2016, reached a more favourable conclusion.
Dr Howick agrees that placebo effects are quite small in general, representing maybe only one or two points difference on a 10-point symptom scale. But these points are clinically relevant, and can make a world of difference in some contexts, he argues.
Imagine a scenario where a doctor saves a patient from opioid addiction by swapping pain medication for an equally-effective placebo, or where a placebo vitamin was prescribed for a viral cold instead of an antibiotic.
In Dr Howick’s systematic review, open-label placebo trials had a moderate effect size of 0.88 (measured as the standardised mean difference/Cohen’s d).
If that statistic makes your eyes glaze over, you can convert it into “numbers needed to treat’ (NNT), which is much simpler to interpret.
In this instance, a doctor would need to treat about five to 10 patients with an open-label placebo to elicit a clinically-meaningful symptom improvement in one patient.
However, most patients would get some benefit from the placebo effect, unless they had been conditioned to respond negatively to medical treatment (which is known as the nocebo effect), Dr Howick said.
The NNT for open-label placebos fares well against that of conventional medications. For example, you would have to treat 66 patients with statins for 3.5 years to significantly reduce the risk of myocardial infarction in one patient, and you would have to treat 111 patients with statins for the same period of time to reduce the risk of stroke in one patient.
Systematic reviews are supposed to get us closer to the truth by assessing whether a particular finding has been replicated across the literature. But it’s difficult to know what to believe when two meta-analyses produce conflicting results.
Sometimes meta-analytical differences can be resolved by looking for obvious flaws in the methodology, or by uncovering hidden conflicts of interest.
Sometimes they can’t. This is because meta-analyses retain an element of arbitrariness no matter how refined and rational the method, says Jacob Stegenga, a philosopher of science at the University of Cambridge and the author of the book, Medical Nihilism.
Researchers will make different decisions about which studies to include in the meta-analysis, and how to weight the results of each study, and there’s often no rational way to adjudicate between these different approaches.
“Meta-analysis, when done well, is a valuable method in medical research,” says Dr Stegenga. “Nevertheless, the general epistemic importance given to meta-analysis is unjustified, since it is so malleable.”
Dr Stegenga says we may be better served in placebo research by an older tradition of evidence, associated with the English epidemiologist Sir Austin Bradford Hill whose research helped link lung cancer and smoking in the 1950s.
Instead of performing giant meta-analyses, Sir Austin built a case for causality using diverse evidence, including dose-response relationships, temporality, plausible biological mechanisms, coherence with other relevant knowledge, and specificity of causes.
One downside to this approach is that there is no structured method for assessing, quantifying, and amalgamating the very disparate kinds of evidence that Sir Austin considered, says Dr Stegenga.
But a good first step towards proving the potency of placebos would be to demonstrate dose-responsiveness, he says. This could be easily done, for example, by giving some patients instructions to take a placebo pill once a day, and giving others instructions to take placebo pills two, three, or four times a day – and then measuring the magnitude of the effect. “I haven’t come across such a trial,” says Dr Stegenga.
There are hints of dose-responsiveness in placebo-controlled RCTs, as the length of the study seems to influence the power of the placebo.
The next step in the Sir Austin strategy is to demonstrate a credible mechanism for action.
Mechanistic explanations for placebos that include the words “mind-body self-healing” may induce minor nausea if you’re a strictly evidence-based health practitioner. But it seems that the placebo effect is not exclusively grounded in magical thinking. There is quite a lot of serious neuroscience behind it.
Medical rituals are thought to activate the reward mechanism in the brain, releasing dopamine and endorphins, and may jump-start biological processes, such as repair and digestion, by dialling down stress and anxiety.
The best-studied example of this is placebo analgesia, which is mediated by changes in neurochemistry and neural activity in several distinct areas of the brain that amplify and inhibit incoming pain signals.
The mechanisms behind the placebo’s effect on conditions such as allergic rhinitis and IBS are more sketchy. But, given that the sympathetic and parasympathetic nervous systems have significant psychological components, it is not entirely implausible that the subconscious mind could modulate biological processes related to symptom relief.
While the use of honest placebos in clinical practice is uncommon, deceptive placebo use appears to be widespread among doctors worldwide. A 2013 survey of around 800 GPs in the UK found that 97% had prescribed “impure placebos”, or real medications that were unlikely to have an impact on the patient’s condition (such as antibiotics for a viral cold).
A study in 2008 found that 55% of internists and rheumatologists in the US reported using a pure or impure placebo. Around 29% of German physicians have prescribed a placebo at least once, according to a 2016 survey.
The Australian Medical Association is quiet on the topic of placebo prescriptions.
But the American Medical Association supports the use of placebos where the doctor enlists the patient’s cooperation, obtains the patient’s general consent to administer a placebo, and avoids giving a placebo merely to mollify a difficult patient.
In a 2011 report called Placebo in der Medizin, the German Medical Association recommended that doctors consider prescribing placebos for minor ailments where there is no better treatment available.
Seven years on, that position hasn’t changed. “Experimental research on the placebo effect has shown that this effect is part and parcel of every treatment and that doctors should be advised to make as much use of it as possible in their clinical practice,” Professor Robert Jütte, a medical historian and spokesperson for the German Medical Association, told The Medical Republic.
Perhaps the most compelling evidence that expectations really can change reality is high consumer demand for expensive placebos.
Prior to action from the ACCC in 2016, customers frequently paid a premium for Nurofen that promised to alleviate particular kinds of pain, such as back pain, period pain, migraine pain and tension headaches, even though the active ingredient (ibuprofen lysine 342mg) was the same as cheaper products.
Homeopathic products are essentially bottled water, and yet Australians spent around $8 million on homeopathy in 2009, according to the World Health Organisation.
Vitamins, herbs and mineral supplements have not been shown to be superior to placebos for most indications, but Australia’s complementary medicine industry generated $4.9 billion in revenue in 2017 and served 8.1 million regular customers.
Alternative medicine is “unrestrained by the laws of normative physics” and offers exaggerated expectations and magical anticipation, which heightens the “placebo drama”, says Professor Kaptchuk.
“Complementary medical practitioners are much better at evoking the placebo effect than conventional medical practitioners,” says Dr Howick. “They have a nice, clean space with relaxing music playing. They will talk to you for half an hour, whereas when you go to a conventional doctor, they are busy, they are overloaded with paperwork, they have 10 minutes to spend with you – you are in and out.”
However, general practice has its own rituals and symbols that can be leveraged to maximise the placebo effect, says Dr Howick. Everything about a doctor’s office infuses the patient with confidence in the doctor’s healing powers, he says.
There’s the out-of-pocket cost, the degree on the wall from some prestigious university, the white coat, the doctor’s authoritative manner, the stethoscope, the diagnostic process – and, finally, the pill prescription.
“These are all rituals that can start to induce the healing response,” says Dr Howick.
References:
Systematic reviews
Effects of placebos without deception compared with no treatment: a systematic review and metaanalysis (2016)
Placebo interventions for all clinical conditions (Cochrane review 2010)
Open-label placebo trials
Placebos without Deception: A Randomized Controlled Trial in Irritable Bowel Syndrome (2010)
Open-Label Placebo for Major Depressive Disorder: A Pilot Randomized Controlled Trial (2012)
Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial, Scientific Reports (2018)
Open-label placebo treatment in chronic low back pain: a randomized controlled trial (2016)
Altered Placebo and Drug Labeling Changes the Outcome of Episodic Migraine Attacks (2014
Why do open-label placebos work? A randomized controlled trial of an open-label placebo induction with and without extended information about the placebo effect in allergic rhinitis (2018)
Application of open-label placebos in clinical practice
What techniques might be used to harness placebo effects in non- malignant pain? A literature review and survey to develop a taxonomy (2017)
Half of all German doctors prescribe placebos, new study shows (2011)
The Placebo Effect in Alternative Medicine: Can the Performance of a Healing Ritual Have Clinical Significance? (2002)