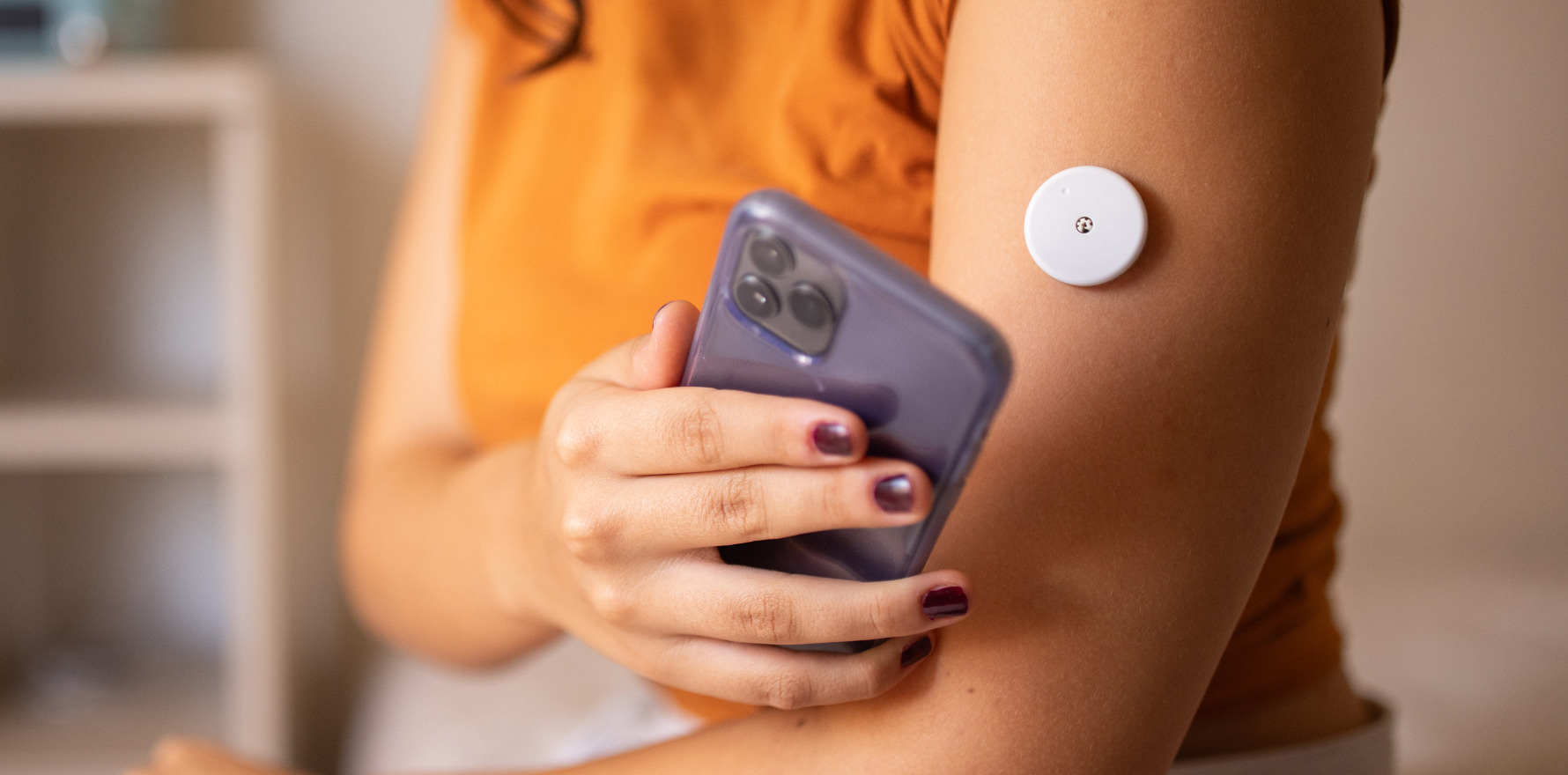
Meanwhile Australian GPs are only allowed to prescribe them to veterans.
News that the US FDA has approved the first over-the-counter CGM should come as a kick in the pants for Australian regulators.
Overnight the Food and Drug Administration cleared for marketing the OTC Dexcom Stelo Glucose Biosensor System, an integrated CGM intended for anyone 18 years and older who does not use insulin.
The Stelo Glucose Biosensor System uses a wearable sensor, paired with an application installed on a user’s smartphone or other smart device, to continuously measure, record, analyse and display glucose values in people 18 years and older who are not on insulin and who do not have problematic hypoglycaemia.
Users can wear each sensor up to 15 days before replacing with a new sensor. The device presents blood glucose measurements and trends every 15 minutes in the accompanying app.
“Giving more individuals valuable information about their health, regardless of their access to a doctor or health insurance, is an important step forward in advancing health equity for US patients,” said Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health.
“CDRH will continue to support innovation that addresses health equity by moving care and wellness into the home setting.”
Here in Australia, DoHAC continues to prevaricate over what type of diabetics can get a subsidy for their continuous glucose monitors, while GPs can only prescribe CGMs for veterans.
Meanwhile, last week in a public hearing of the federal Inquiry into Diabetes in Australia, a DoHAC official flagged that the department was considering extending subsidies for CGMs to include patients with type 2 diabetes, as well as those with type 1 diabetes, even though it might cost the government over a billion dollars.
Related
Australian GPs can now approve access to subsidised CGMs for DVA card-holders, even though they still cannot prescribe them for anyone other group of people with diabetes.
For non-DVA card holders, the NDSS only allows subsidised access for type 1 diabetics and currently blocks GPs from approving access, a position hotly contested by the RACGP.
In other diabetes news, Global Data reports that the market for glucagon-like peptide-1 (GLP-1) medications aimed at weight management – like Ozempic and Wegovy – could potentially hit US$100 billion in 2030, impacting the sales of CGMs and insulin pumps.
GlobalData’s latest report, Insulin pumps and continuous glucose monitors market forecast to 2033, reveals that the insulin pumps and CGM market is poised to grow at a compound annual growth rate of 6.3% from $11.3 billion in 2023 to $20.9 billion in 2033.
“While GlobalData does not foresee a significant risk from GLP-1 medications on the insulin pumps market in the short-term, the long-term impact tends to be a bit gloomy,” said GlobalData’s principal medical devices analyst Tina Deng.
“GLP-1 medications can help to improve blood sugar control and reduce the need for additional insulin in patients with type 2 diabetes. It can also delay the need for insulin and limit progression for patients with type 2 diabetes who have not taken insulin yet.
“However, as the device penetration of insulin pumps is less than 1% of type 2 diabetes patients, the overall impact seems to be more moderate.”